![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
|
Formula of benzene |
C6h6 |
|
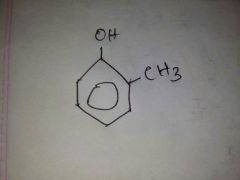
Name this |
2 methyl phenol |
|
|
Why kekule model failed |
1. All bonds were of the same length, between that of single and double bond 2. Absorbs ir energy at different frequency from that of single and double bond |
|
|
The ring of e is caused by |
Delocalized electron form p orbital |
|
|
What can we conclude about the difference in theoretical and actual enthalpy change in hydrogenation of benzene |

|
|
|
How benzene burns |
With yellow smoky flame |
|
|
Type of reaction that benzene mostly undergoes |
Electrophilic substitution |
|
|
Practical steps for preparation and collection of nitrobenzen |
Warm with reactants. Notrobenze floats on top. |
|
|
Does nitration of benzene have change of multiple nitration? If yes, state ways to prevent it |
Yes .. Keep temperature below 55 |
|
|
Practical steps of sulphonation And temp |
Warm with oleum at 40 |
|
|
Condition for friedel craft alkylation |
Alcl3 is sensitive to hydrolysis. So the reaction mixture is refluxed in dry ether. |
|
|
Name of product of friedel craft acylation |
Phenylketone |
|
|
Risk of multiple acylation ...... |
No. Because phenyl ketones are less reactive . |
|
|
Benzenes addition reaction |

|
|
|
Methyl oxybenze |
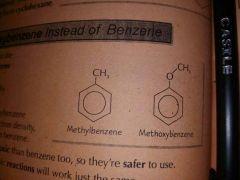
|
|
|
Reactivity of methyl benzene and and methyloxybenze compared to benzen |
They are much more reactive |
|
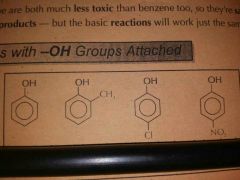
Name these |
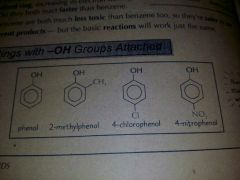
|
|
|
Equation for reaction between phenol and bromine. Name the products. |
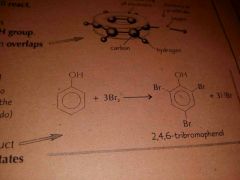
|
|
|
Conditions for reaction between phenol and bromine. |
None. Its much more reactive. |
|
|
Why phenol is more reactive that benzene |

|
|
|
Reagents for nitration of phenol |
Dilute nitric acid |
|
|
Products of nitration of phenol |
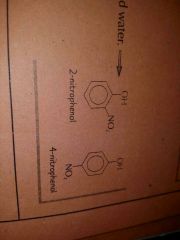
|
|
|
Nitration of benzene vs phenol |

|
|
|
Colour and solubility of tribromophenol |
White insoluble solid |

