![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
359 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Case-Control Study
Definition and variable measured: |
1. A retrospective comparison of two groups -- a control group and a disease group -- searching for the existence of a risk factor or factors.| - i.e. smoking histories are compared in a group of women with breast cancer to those in a group of women without breast cancer.||2. Odds ratio -- i.e., that one group will have it, vs. odds that the other will have it.| - Women with breast cancer are twice as likely to have smoked in the past than women without it.
|
|
|
|
BAX
(1) Function (2)Path |
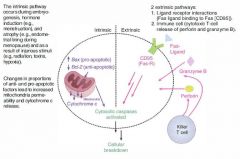
(1) This enzyme of the intrinsic pathway to apoptosis functions to segregate BCL-2, permitting cytochrome C to escape from the mitochondrial membrane and activate caspases.
BAX is upregulated by p53. |
(2) When p53 is knocked out by mutation, BAX cannot be stimulated, and BCL-2 protects the cell from apoptosis indefinitely, resulting in cell immortality.
|
|
|
(1) What are the proportions of lipid-subtypes in the cell membrane?
(2) How does cholesterol effect the structure of a lipid membrane? |
(1)
50% phospholipids 50% cholesterol Traces of others |
(2)
Cholesterol stiffens and weakens the bilipid membrane because it interferes with the fluid interaction of phospholipids. |
|
|
Cohort study|Variable measured:
|
Relative risk (RR).| - i.e., our cohort study of two groups of patients, smokers and non-smokers, revealed a 5-fold risk of developing lung cancer.| - Answers the question: "what will happen?"
|
|
|
|
BCL-2
(1) Function: (2) Path: |
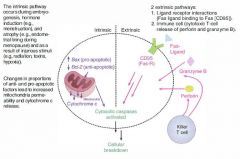
Function:
This anti-apoptotic enzyme functions to prevent cytochrome C from escaping the mitochondria and causing caspase activation. |
Path:
The t(14:16) translocation moves the BCL-2 gene so that it's adjacent to and co-expressed with the Ig heavy chain gene. Result: lots and lots of BCL-2, no apoptosis, and therefore follicular lymphomas (ALL). |
|
|
Location of Vimentin
|
Intermediate filament found in connective tissue
|
|
|
|
Phase 1 of clinical trials|Design and objectives:
|
Prospective study with control group using a **small number of healthy subjects.**| - Objective: determine the toxicity, safety, and pharmacokinetics of the drug.
|
|
|
|
What are the 2 extrinsic pathways to apoptosis?
|

(1) Ligand-receptor interactions:
Fas ligand binds to Fas [CD95] on cell surface, triggering caspase activation. |
(2) Cytotoxic T-cell pathway:
1. CD8+ T cells recognize tumor or viral antigen and secrete perforin to punch holes through the problematic cell 2. CD8+ T cells then secrete granzyme, which enters through those holes and activates caspases |
|
|
Location of Desmin
|
Intermediate filament found in muscle
|
|
|
|
Phase 2 of clinical trials|Design and objectives
|
Prospective study with control group using a small number of sick patients.| - Objective: determine efficacy, optimal dosing, and adverse effects.
|
|
|
|
What types of hypoxic cellular injury are (1) reversible, and (2) irreversible?
|
(1) Reversible hypoxic injury ~ membrane swelling.
1. ATP production slows d/t lack of O2 to run the ETC. Intracellular hyperosmolality and swelling occur as a result of: --a. Na/K ATPase slows down --b. Calcium ATPase slows down --c. Lactic acid fermentation results in increased H+ in the cell (and lactic acidosis, more globally) 2. Blebbing occurs when swelling pulls plasma membrane away from the cytoskeleton. --Results in microvilli effacement, just as inflating a surgical glove results in shrinking of the fingers. 3. RER swelling occurs for the same reasons, resulting in ribosome detachment and decreased protein synthesis --B/c ribosomes are attached by weak, non-covalent bonds to the RER. |
(2) Irreversible damage
Inner mitochondrial membrane damage --releases cytochrome C. There's no recovering from this. 1. Plasma membrane damage --this is why troponin is a good indicator of irreversible damage (infarct!) 3. Lysozomal membrane rupture releases proteases, etc., that autodigest the cell. |
|
|
Location of Cytokeratin
|
Intermediate filament found in epithelial cells
|
|
|
|
Phase 3 of clinical trials|Design and objectives:
|
Prospective study using a large number of healthy patients, randomly assigned to the test and control groups.| - Objective: compare new treatment to current standard of care.| - Control group can be given a different, established treatment or a placebo.
|
|
|
|
What nuclear changes do we see in (1) reversible hypoxic injury and (2) irreversible hypoxic injury?
|
(1) this injury is accompanied by reversible clumping of chromatin.
|
(2) This injury is accompanied by (in order):
1. Pyknosis: irreversible chromatin condensation 2. Karyorrhexis: blebbing of nucleus into vacuoles in the cytoplasm 3. Karyolysis: dissolution of chromatin in dying cells resulting in the characteristic finding of coagulation necrosis: no nuclei and diffuse pink staining. |
|
|
Location of GFAP
|
Intermediate filament found in found in Neuroglia
|
|
|
|
Phase 4 of clinical trials|Design and objectives:
|
Postmarketing surveillance trial of patients after approval of drug.| - Detects rare or long-term adverse effects.
|
|
|
|
Which part of the colon is most susceptible to hypoxic injury?
|
Splenic flexure of the Large Intestine, b/c it is the watershed region supplied by the terminal superior and inferior mesenteric circulations.
|
|
|
|
Location of Neurofilaments
|
Intermediate filament found in neurons.
|
|
|
|
How does knowing the 5 intermediate filaments help us?
|
Because they're very large and abundant cytoskeletal structures, a stain specific for one of them will light up the tissue it lives in.
|
|
|
|
Formula for sensitivity
|
True positives / (true positives + false negatives)||True positives / Total positives
|
|
|
|
What organs undergo (1) red/hemorrhagic infarct, and (2) pale infarct?
|
(1) Soft organs supplied by dual circulation:
-- Lungs, Liver, Intestine -- Or any organ that is reperfused after infarct. |
(2) Solid tissues with a single blood supply, i.e.:
-- Heart, Kidneys, Spleen |
|
|
Ouabain
Mech: |
This toxin is found in leaves and bark in Africa and was used to poison arrow and spear-tips.
It blocks the K+ side of Na/K ATPase. |
|
|
|
Formula for specificity
|
True Negatives / (true negatives + false positives)||True negatives / Total negatives
|
|
|
|
Give an example of each of the 2 kinds of shock.
|
(1) Low output failure: can be due to:
-- Hypovolemia, i.e. in dehydration -- Cardiogenic, i.e. in dilated cardiomyopathy. -- accompanied by cold extremities, low TPR (2) Hi output failure: can be due to: -- early sepsis: tachycardia -- accompanied by warm extremities, decreased TPR, lots of volume lost to interstitial space. |
|
|
|
Cardiac glycosides
Mech: |
Directly inhibit Na/K ATPase (slightly), decreasing the cells ability to pump out Ca2+.
Result: increased intracellular Ca2+ = increased cardiac contractility! |
|
|
|
Formula for PPV
|
True Positives / (True positives + False positives)||True positives / Total positive test results|| = the probability that a positive test result is correct.
|
|
|
|
Clinical symptoms of inflammation:
|
Rubor
Dolor Calor Tumor functio laesa (loss of function) |
|
|
|
Where are types II and III collagen found?
|
Type II collagen: found in cartilage.
Type III collagen: found in blood vessels, lymph nodes, dermis, and early wound healing. AKA reticulin |
|
|
|
Formula for NPV
|
True negatives / (True negatives + False negatives)||True negatives / Total negatives|| = the probability that a negative test result is correct.
|
|
|
|
Molecular components of "Rolling" in leukocyte extravasation
|
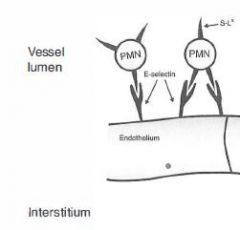
On leukocytes: Sialyl Lewis (glycoprotein)
On Endothelium: E- and P-Selectins |
|
|
|
Ehler Danlos?
Path & Presentation: |
Path: Type III collagen is defective d/t alpha chain mutation.
|
Presentation: joint hypermobility, hyperelastic skin, heart valve issues
|
|
|
In chronic disease, which is greater: prevalence or incidence?
|
In chronic disease, prevalence is greater than incidence because a low rate of new diseases can still result in a relatively hi prevalence.
|
|
|
|
In the "Tight Binding" stage of leukocyte extravasation, what molecular components are interacting?
|
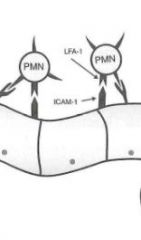
Leukocyte: LFA-1 (integrin)
Endothelium: ICAM-1 |
|
|
|
Types of Collagen disorders:
|
Type I: Osteogenesis imperfecta, scurvy
Type III: Ehler Danlos Type IV: Alport's (defective) & Goodpastures (auto-antigenic) |
|
|
|
Difference between Odds Ratio and Relative Risk
|
Odds ratio refers to the relative odds that a patient with a risk factor for a disease will also have that disease *right now*.| - It is determined via Case-Control study.||Relative risk refers to the risk that this patient will contract that disease in the future.| - It is determined by Cohort study.
|
|
|
|
In the Diapedesis stage of leukocyte extravasation, what molecular components are at work?
|
PECAM-1 proteins on both leukocytes and endothelium are interacting, here...
|
|
|
|
Describe Collagen Structure:
|
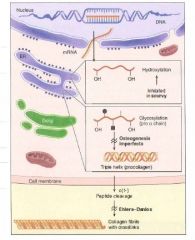
Triple helix made of Gly-X-Y repeats!
X and Y can be proline, hydroxyproline, or hydroxylysine! The Glycine residues line up axially, stabilizing the structure by their hydrophobic interactions. |
|
|
|
Define "Number needed to harm"
|
1 / Attributable Risk
|
|
|
|
After successful extravasation from the blood vessel, how is the leukocyte directed to its target?
|
CILK:
C5a IL-8 Leukotriene B4 Kallikrein (protease that converts HMW kininogen to bradykinin) |
|
|
|
Steps of Collagen Synthesis:
|
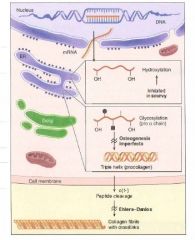
(1) Intracellular steps:
1. Translation of Gly-X-Y polypeptide. [preprocollagen] 2. Hydroxylation of lysin and proline residues (requires vitamin C) 3. Glycosylation of hydroxylysine residues |
(2) Extracellular steps:
4. Proteolytic processing: cleavage of terminal regions of procollagen [Tropocollagen, the basic monomer] 5. Tropocollagen strands link to eachother via lysine-hydroxylysine crosslinkages for enhanced strength. [Collagen fibril] |
|
|
Recall bias
|
Occurs when the patient's knowledge that he has a disease alters his answers to study questions.
|
|
|
|
What phase of drug metabolism is sometimes responsible for the production of toxic compounds?
|
Phase I drug metabolism occurs in the liver and is carried out by CYP450 enzymes, NADPH, and oxygen.
Phase I are *non-synthetic* reactions, including oxidation, reduction, hydrolysis, cyclization and decyclization reactions. They can sometimes toxify otherwise harmless substances. |
Contrast with Phase II *conjugation* reactions, which are usually detoxifying in nature.
These reactions include: -- methylation by methyltransferases -- acetylation by acetyltransferases -- glycosyluronidation -- etc. |
|
|
Steps of Collagen Synthesis:
|
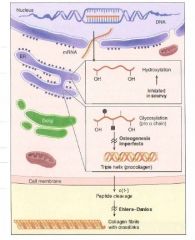
(1) Intracellular steps:
1. Translation of Gly-X-Y polypeptide. [preprocollagen] 2. Hydroxylation of lysin and proline residues (requires vitamin C) 3. Glycosylation of hydroxylysine residues |
(2) Extracellular steps:
4. Proteolytic processing: cleavage of terminal regions of procollagen [Tropocollagen, the basic monomer] 5. Tropocollagen strands link to eachother via lysine-hydroxylysine crosslinkages for enhanced strength. [Collagen fibril] |
|
|
Late-look bias
|
Occurs when patients who have passed away from an illness are therefore excluded from a study of that illness.
|
|
|
|
List the causes of free radical damage other than UV radiation exposure.
|
1. Drug metabolism (Phase I)
2. Nitric oxide 3. Redox reactions 4. Transition metals 5. Leukocyte oxidative burst |
|
|
|
How does intracellular collagen differ from extracellular collagen? Why must we finish making this stuff outside the cells?
|
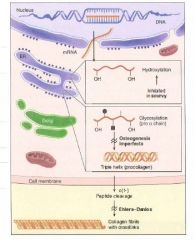
As pro- and preprocollagen, the molecule is still soluble.
Once the hydrophilic end segments are cleaved in the RER lumen, the molecule becomes insoluble, and would be lethal to cells. |
|
|
|
Pygmalion effect:
|
Occurs when a researcher's beliefs regarding the efficacy of a treatment effect the outcome of that treatment| - i.e., the researcher gives more attention to patients who are getting the treatment than to the placebo group.| - compare to Hawthorne effect, in which the subjects are the source of the bias.
|
|
|
|
List the 4 enzymes that reduce free radical damage.
|
1. Catalase:
- found in peroxisomes, generates H2O2 used to neutralize free radicals - also a virulence factor found in bacteria. 2. Superoxide dismutase & Myeloperoxidase: - Macrophages use these to generate the H2O2 and CLO- radicals of the oxidative burst, which allows them to digest what they've eaten. 3. Glutathione peroxidase - found in liver, exhausted Tylenol overdose. |
|
|
|
What are the most common and most deadly forms of osteogenesis imperfecta? How do they differ?
|
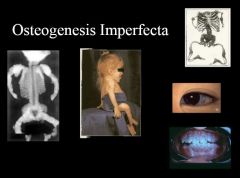
Osteogenesis Imperfecta Type I:
Mildest form Autosomal dominant Osteogenesis Imperfecta Type II: autosomal recessive Lethal, perinatally |
|
|
|
Define "Power".
|
Power is the probability that when a study claims its findings differ from the null hypothesis, they actually do.
It can also be phrased as the probability of rejecting the null hypothesis when it is in fact false. = 1 - beta, or 1 - type II error |
|
|
|
What prominent symptom of several different lysozomal storage disorders is caused by free radical damage?
|
Retinopathy of prematurity: a retinopathy that can be accompanied by excessive O2 therapy in premature infants with too little surfactant.
Often accompanied by ARDS. |
|
|
|
Steps of PCR:
|
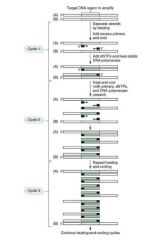
1. Denaturation:
Add lots of mRNA primers, dNTPs, and heat-stabile DNA Polymerase from geyser bacterium to your sample, and heat to denature it. 2. Reanneal: cool enough for RNA primers to anneal, and for special DNA-P to do its work. Repeat, repeat, repeat |
Concept: we use RNA primers that we know to be flanking the gene we want to amplify.
After several cycles, we'll have tons of DNA from that gene only. |
|
|
Define Type I Error in clinical studies.
|
This error occurs when the researcher claims that there is a significant difference between the experimental hypothesis and null hypothesis, but there is not one.
|
|
|
|
Mechanism of leukocyte burst?
In what cells does it occur? |
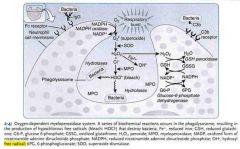
1. NADPH + O2 --NADPH Oxidase--> NADP + O2- [superoxide!]
2. O2- --Superoxide dismutase--> H2O2 3a. H2O2 --Myeloperoxidase--> CLO- [hypochlorite = bleach; very powerful!] -- this is the leukocyte burst 3b. H2O2 + GSH ---> H2O + GSST (inactive metabolite of used-up glutathione). -- this is the protective mechanism by which we get rid free radicals (esp in liver) |
This process is used to kill phagocytized materials in neutrophils and monocytes *only*. *Not* macrophages.
|
|
|
How do we visualize our PCR results?
|
This lab test is visualized using gel electrophoresis on agarose gel.
Shorter pieces of DNA travel further. We then compare the gel against a DNA ladder. |
|
|
|
Define Type II error
|
Occurs when the researcher claims there was no difference between the experimental hypothesis and the null hypothesis.
|
|
|
|
What pathology is associated with inheritance of a defective Glucose-6-phosphate dehydrogenase gene (G6PD)?
|
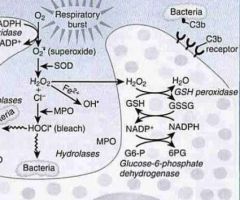
This genetic deficiency results in inability to renew spent NADP+ to NADPH by reducing it with Glucose-6-Phosphate.
Result: Hemolytic anemia aggravated by antimalarial drugs and any other source of radical damage. -- Because G6PD is the only source of NADPH in RBCs, this deficiency causes them to be particularly susceptible to free radical damage. -- No G6PD ==> no NADPH ==> no GSH |
|
|
|
Which Blotting Technique does what?
|
SNoW
DRoP Southern Blot: used to separate DNA Northern Blot: used to separate RNA Western Blot: used to separate Protein |
|
|
|
Define Gaussian
|
The type of distribution in which mean = median = mode
|
|
|
|
What is the biochemical mechanism of reperfusion injury?
|
Free radical injury
|
|
|
|
Materials for a Laboratory Blot:
|
1. DNA/RNA/Protein is electrophoresed on agarose gel, then transferred to a filter.
2. Denaturant is applied and radiolabeled primers are added [nucleic acid primers for Noorthern and Southern blots, and antibody primers for Western blot] 3. Read by exposing to film |
|
|
|
Define positive skew
|
Mean > median > mode
- Distribution in which there is a tail on the positive side of the curve. |
|
|
|
What are the causes of transudates and exudates, respectively?
|
Transudates: hi hydrostatic pressure, low oncotic pressure in the serum compartment. (low osmolarity in the interstitium)
Exudates: blocked lymphatics or inflammation (hyperosmotic interstitium) |
|
|
|
Laboratory Blot Techniques:
|
1. DNA/RNA/Protein is electrophoresed on agarose gel, then transferred to a filter.
2. Denaturant is applied and radiolabeled primers are added [nucleic acid primers for Noorthern and Southern blots, and antibody primers for Western blot] 3. Read by exposing to film |
|
|
|
Which measurement of central tendency changes the least as skew increases?
|
Mode changes the least, b/c the value that corresponds to the greatest number of subjects doesn't really move much.
|
|
|
|
How does microarray work?
|
We immobilize thousands of DNA fragments from known genes on a chip, then add radiolabeled primers from our patient sample.
I have no idea how we get radiolabeled primers from a patients blood/CSF/urine sample, but we do. |
|
|
|
What information do we get from standard deviation and standard error?
|
The former tells us how widely a population varies.||The latter gives us an estimate of the quality of the sample.
|
|
|
|
List the granulomatous diseases.
|
1. TB
2. Crohn's 3. Treponema Pallidum (syphilis) 4. Histoplasmosis (and other fungal infections) 5. M. leprae (leprosy 6. Bartonella henselae (cat scratch fever) 7. Sarcoidosis 8. Berylliosis 9. Takayasu arteritis (large vessel, i.e. aortic arch) 10. Giant Cell Arteritis (temporal and ophthalmic arteries) 11. Wegener's granulomatosis (medium-sized vessel: lung and renal) |
|
|
|
How do ELISA's work?
|
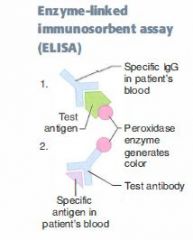
If we're testing for the presence of an antibody in the patient's serum, add its antigen with peroxidases specially attached.
If we're testing for presence of an antigen in the patient's serum, add specific antibodies with peroxidases attached to the sample. Either way, if the patient is sero-positive, there'll be an intense color reaction. |
|
|
|
Standard deviations for the 95th and 99th percentiles?
|
95th percentile = 2.standard deviations|99.7th percentile = 3 standard deviations
|
|
|
|
Biochemistry of granulomatous inflammation:
|
1. Th1 cells at the site secrete INF-gamma, causing macrophage chemotaxis.
2. Macrophages at the site secrete TNF-alpha, inducing and maintaining granuloma formation. 3. Multinucleate giant cells form. |
|
|
|
Pleiotropy
|
1 gene has more than 1 effect on an individual's phenotype.
i.e. PKU: it's a monogenic disorder, but causes MR, hair and skin changes, and a bunch of other seemingly unrelated problems. |
|
|
|
What percentage of a population fits within 1 standard deviation of the mean?
|
68% of the population falls within 1 standard deviation of the mean.
|
|
|
|
What is erythrocyte sedimentation rate, and what does it tell us?
|
This serological datum measures the amount of time it takes for RBCs to coagulate and precipitate out within a test tube.
Products of inflammation bind to RBCs, causing aggregation and increasing ESR. |
|
|
|
Loss of heterozygosity
|
The 2nd hit of the 2-hit hypothesis.
|
|
|
|
Which statistical test would we use to compare the means of two different populations? How about 3 different populations?
|
For the former, we use a t-test|For the latter, we use ANOVA (Analysis of Variance)
|
|
|
|
What are the consequences of elevated / decreased ESR?
|
Elevated ESR:
-- Infections -- Temporal arteritis -- Cancer -- Pregnancy -- SLE In inflammatory states, hi presence of fibrinogen causes clotting/sedimentation rate to increase |
Decreased ESR:
-- Sickle cell (altered shape) -- Polycythemia (too many) -- CHF (unknown) |
|
|
Dominant negative mutation
Give an example |
When a heterozygote produces 1 mutant allele that doesn't only fail to work, but also interferes with the function of the other, normal allele.
|
Example: a mutation of a Transcription factor allele in its allosteric site: allele can still bind its target, blocking out the good copy of the TF from acting!
|
|
|
Equation for Power
|
1 - beta||Beta = probability that null hypothesis was confirmed mistakenly by results of research.
|
|
|
|
What form of poisoning is a common cause of free radical damage?
|
Iron poisoning is one of the most common causes of poison-induced fatality in children.
the free radical burden it causes damages membranes. |
|
|
|
Lyonization
|
Random X inaction in females, resulting in one normal X chromosome and one Barr body.
Perfectly normal, but it means that any mutations on the normal X chromosome will be dominant. |
|
|
|
Equation for Confidence Interval
|
Saying that C.I. = 95% is the same as saying that we expect 95% of the population to fall within 2 standard error measurements of the mean in either direction.||CI = [mean - Z(SEM)] through [mean + Z(SEM)]||SEM = Standard deviation / (square root of n)
|
|
|
|
How do we identify amyloid deposits in diagnostic testing?
|
These deposits display apple-green birefringence of Congo Red stain under polarized light.
|
|
|
|
Locus heterogeneity
|
When mutations at two different loci cause the same phenotype.
|
Occurs in Marfan's, MEN 2B, and homocystinuria, all of which result in clinical marfan's syndrome.
|
|
|
How do we use the chi-square test?
|
The chi-square test is used to check the differences between 2 or more percentages or proportions of categorical outcomes.| - i.e., tests the difference between proportions of pt's with bodyweight > 140 between groups A, B, and C at a given time.
|
|
|
|
Bence Jones
(1) Protein & its source: (2) Associated pathology: |
Protein making up deposits: AL
Derived from: Ig light chains |
Amyloidosis seen in multiple myeloma
|
|
|
Heteroplasma
|
Inheritance of both normal and mutant mitochondrial DNA (mtDNA), resulting in variable expression of mitochondrial disease.
|
Depends upon which mitochondria were inherited by the lucky germ cell!
|
|
|
What are the degrees of disease prevention?
|
PDR:
1st degree: Prevention, i.e. via lifestyle changes 2nd degree: Early diagnosis, i.e. by pap smear 3rd degree: Reduction of disability, i.e. by cytoxin treatment! |
|
|
|
Achondroplasia
Etiology and biochem Inheritance pattern |
FGFR3 (fibroblast growth factor 3) defect resulting in dwarfism, short limbs, but head and trunk are normal size.
Autosomal-dominant |
Autosomal Dominant
|
|
|
What is a "living will"?
|
This term designates a written advanced directive specifying what sorts of treatments and procedures we are to receive or not receive in the event that the patient becomes incapacitated and cannot speak for himself.
|
|
|
|
Secondary Amyloidosis
(1) Protein and it's source: (2) Associated pathology |
Protein: AA
Derived from: Serum amyloid-associated protein |
Amyloidosis found in chronic inflammatory disease
|
|
|
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
[the adult form] Etiology: |
Always bilateral, massive enlargement of kidneys d/t multiple large cysts.
Presents with flank pain, flank swelling, hematuria. |
Autosomal dominant
**Associated with berry aneurisms An infantile recessive form exists that's much worse. |
|
|
How much should we tell a curious child about her illness?
|
In this situation, the parents make the determination, not us.
|
|
|
|
Transthyretin deposits
Pathology & its precursor: |
Found in senile cardiac amyloidosis
Derived from: AF protein [Old *Fogies] |
|
|
|
Familial Hypercholesterolemia
|
Elevated LDL d/t defective or absent LDL receptor.
Presents with MI, very hi cholesterol. Homozygotes are rare and don't live long. |
Autosomal Dominant
|
|
|
What are the parameters of an Apgar assessment?
|
This assessment of newborns is made at 1 and 5 minutes after birth. It scores the child's health based on:
1. Appearance - coloration of body and extremities, signifying quality of ventilation 2. Pulse 3. Grimace (reflex irritability): - poke child on foot, 1 point for a grimace, 2 for withdrawal of foot 4. Activity (muscle tone) - no motion, weak motion, strong kicking 5. Respiration - no breathing, weak/slow breathing, rapid breathing & crying. |
|
|
|
Amylin deposits
Pathology & its precursor: |
Found in amyloidosis in DM2
Derived from: AE [*Endocrine] |
|
|
|
Familial Adenomatous Polyposis
|
Colon becomes covered in polyps. Colorectal cancer is inevitable.
APC mutation on chromosome 5. [5 letters in polyp] |
Autosomal Dominant
|
|
|
How is an Apgar assessment interpreted?
|
The APGAR assessment, given once at 1 minute and once at 5 minutes, each time gives us a score between 1 and 10 for a newborn child.||Interpretation of results:| - 10-7 is normal| - 4-6 = "assist and stimulate"| - Less than 4 indicates imminent danger of death.
|
|
|
|
A-CAL
|
Found in amyloidosis secondary to Medullary Carcinoma of the Thyroid
Derived from: Calcitonin |
|
|
|
Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome)
Etiology: Pattern of Inheritance: |
Inherited disorder of blood vessels. Findings include telangiectasia, recurrent epistaxis, arteriovenous malformations.
Presents with purpura, nosebleeds, telangiectasia on tongue. |
Autosomal Dominant
|
|
|
Moro reflex
|
When startled, the child extends arms and legs. This reflex disappears by 3-4 months of age.
|
|
|
|
beta-amyloid
|
Found in amyloidosis in Alzheimer's Disease.
Derived from amyloid precursor protein (APP) |
|
|
|
Hereditary spherocytosis
Etiology: Pattern of Inheritance: |
Spheroid eryhthrocytes d/t ankyrin or spectrin defect.
Presents with hemolytic anemia, increased MCHC. This is the disorder in which the wire mesh of the normal spleen is responsible for your hemolysis. |
Autosomal Dominant
|
|
|
Milestones of early development
0-3 months: |
1. Rooting reflex
2. Moro reflex disappears 3. Can hold head up |
|
|
|
β2-microglobulin
|
Found in dialysis-associated amyloidosis.
Derived from MHC class I proteins. |
|
|
|
Marfan's Syndrome
Etiology: Pattern of Inheritance: |
Fibrillin gene mutation resulting in connective tissue abnormalities.
|
Autosomal Dominant
|
|
|
At what ages do children typically begin to experience stranger anxiety?
|
7-9 months.
|
|
|
|
Cause of metaplasia?
|
Cell change usually environmental exposure.
|
|
|
|
MEN
Etiology: Pattern of Inheritance: |
Familial tumors of the endocrine glands.
Types 2A and 2B are caused by RET mutation. |
All 3 syndromes are autosomal dominant
|
|
|
At what ages do children typically lose the Moro reflex?
|
At 3-4 months, children cease to reflexively extend arms and legs when startled.
|
|
|
|
Define desmoplasia.
|
Defined as fibrous tissue formation in response to neoplasm. Seen in many tissue types, i.e. breast and pancreas.
|
|
|
|
Neurofibromatisis type 1
Etiology: Pattern of Inheritance: |
Cafe Au lait spots
neural tumors blindness Lisch nodules are ubiquitous Mutation on long arm of chromosome 17 |
Autosomal Dominant
|
|
|
At what ages do children typically lose the rooting reflex?
|
At 3-4 months, children cease to reflexively turn their head in the direction of a stroke on the cheek. This reflex serves to bring them to the nipple.
|
|
|
|
Difference between grading and staging:
|
Grading is based upon degree of dedifferentiation of the neoplastic cells, and is recorded as I-IV.
Staging is based upon degree of localization/spread, i.e. bloodborne mets = Stage 4. Staging is more prognostic of outcomes. |
|
|
|
Neurofibromatosis type 2
|
Bilateral acoustic schwannomas
Juvenile cataracts NF2 on Chromosome 22 [2's disease: Type 2 on chrom. 22] |
Autosomal dominant
|
|
|
At what ages do children typically begin to show a social smile?
|
At about 3 months of age, children begin to smile.
|
|
|
|
What staging system is most commonly used at present? How is it used?
|
TNM staging is currently the most commonly used staging scale in diagnosis of neoplasms.
T: describes primary Tumor; it's degree of dedifferentiation, etc. N: degree of spread to regional lymph Nodes M: Presence of Mets |
The method of TNM staging varies for each different type of cancer, but in general it is supposed to tell us about the primary tumor, any regional spread, and any distal metastases.
|
|
|
Tuberous sclerosis
|
Facial lesions
Hamartomatous masses in cortex and retina Increased incidence of astrocytomas |
Autosomal dominant
|
|
|
At what age does a child become able to transfer toys from hand-to-hand?
|
At about 7-9 months of age, children begin to show more complex motor behaviors such as this.
|
|
|
|
What distinguishes an invasive carcinoma from a carcinoma in situ?
|
The invasive carcinoma must make collagenases and/or hydrolases (metalloproteinases) in order to burrow through the basement membrane underlying the epithelium.
|
|
|
|
von Hippel-Lindau disease
|
Hemangioblastomas of retina/cerebellum/medulla.
Half of these individuals also develop renal cell carcinoma. Familial form is caused by VHL gene mutation on p arm of chromosome 3. |
Autosomal dominant
There is also a sporadic form caused by loss of p arm of chromosome 3. |
|
|
At what age does a child begin to walk?
|
Children develop this ability at about 12-15 months of age.
|
|
|
|
Define cachexia. What are its chemical mediators?
|
Loss of weight, muscle atrophy, and fatigue that occur in chronic disease.
Mediated chemically by: -- TNF-alpha (AKA cachectin) -- INF-gamma -- IL-6 |
|
|
|
VHL
(1) Genetics & biomolecular changes (2) Path |
(1)
Gene located on 3p. Can be lost d/t del(3p) or mutation to the VHL gene on 3p. VHL normally degrades the transcription factor HIF-1α, preventing it from triggering PDGF and VEG-2F expression. |
Disease consists of medulloblastomas, hemangiomas of the retina/cerebellum/medulla.
Half of these individuals also will get renal cell carcinoma. Bottom line: when VHL is knocked out, we'll see cancerous expansion of many small blood vessels. |
|
|
At what age do children begin to manifest separation anxiety?
|
Children begin to manifest this emotional response at 12-15 months of age.
|
|
|
|
What neoplasm(s) are associated with Down's Syndrome?
|
1. ALL (we ALL fall DOWN)
2. AML |
|
|
|
Cystic Fibrosis
Normal vs. pathological pulmonary biochem: |
Normal Lungs:
CFTR is a Cl- channel that actively secretes chloride in lungs CFTR regulates ENaC, preventing excessive Na+ absorption by the lung epithelium. |
Lungs in CF:
1. Cl- channel is absent or defective, so less Cl- is secreted from lung epithelium. 2. Cl- channel cannot, under these circumstances, downregulate ENaC activity, and Na+ is reabsorbed into lung epithelium too vigorously 3. H2O follows Na+ out of the airways and into the epithelium 4. Result: thick, stagnant mucous in airways that forms plugs and invites bacterial colonization. so it can't regulate ENaC, and lots of Na+ (and therefore H2O) is reabsorbed. |
|
|
At what age do children begin to develop their core gender identity?
|
Children begin to develop their core gender identity at 24-36 months of age.
|
|
|
|
What neoplasm(s) are associated with Xeroderma Pigmentosum?
|
*1. Squamous cell carcinoma (most of all)
2. Melanoma 3. Basal cell carcinoma |
|
|
|
Genetics of CF & Location of Cystic Fibrosis gene
|
CFTR is located on the long arm of Chromosome 7
Autosomal recessive inheritance pattern |
|
|
|
At what age do children typically begcome capable of riding a tricycle?
|
At 3 y/o, children typically become able to participate in this more complex form of play.
|
|
|
|
What neoplasm(s) are associated with Chronic atrophic gastritis, pernicious anemia, and postsurgical gastric remnants?
|
Gastroadenocarcinoma
|
|
|
|
Complications of Cystic Fibrosis
|
Lung:
1. recurrent infections by pseudomonas and staph aureaus 2. chronic bronchitis, bronchiectasis GI: pancreatic insufficiency |
|
|
|
How many words does a 3 y/o's vocabulary typically contain?
|
At this age, a child can speak about 900 words and can make complete sentences.
|
|
|
|
What is tuberous sclerosis and what are its complications?
|
Syndrome characterized by facial angiofibroma, large hamartomas in the cortical matter, seizures, and MR?
Seizures are the result of mass effects from these big growths. |
1. Astrocytoma
2. Angiomyolipoma 3. Cardiac rhabdomyosarcoma |
|
|
Compare CF pathophys in skin vs. lung
|
In lung, CF is caused by defect in a Cl- channel that normally actively SECRETES Cl-.
In the skin, CF is caused by defect in a Cl- channel that actively REABSORBS Cl- In both cases, pathological buildup of Cl- is mirrored by pathological buildup of Na+ and H2O, because CFTR regulates ENaC. |
|
|
|
How many words does a child's vocabulary typically have between 12 and 24 months of age?
|
At this age, a child typically can speak about 200 words.
|
|
|
|
What neoplasm(s) are associated with actinic keratosis?
|
Squamous cell carcinoma
|
|
|
|
Cystic fibrosis
Normal vs. pathological biochem in skin: |
Normal Skin:
1. CFTR is a Cl- transporter that actively *reabsorbs* Cl- from sweat 2. CFTR upregulates ENaC activity, and therefore Na+ reabsorption. |
Skin in CF:
1. CFRT is absent or defective, so less Cl- is reabsorbed by the skin. 2. Cl- channel cannot regulate ENaC, so Na+ isn't reabsorbed either. 3. Lots of NaCl is lost in sweat. |
|
|
At what point does grieving become considered pathological?
|
more than 2 months.
|
|
|
|
What is Plummer-Vinson syndrome?
What neoplasm(s) are associated with Plummer-Vinson syndrome? |
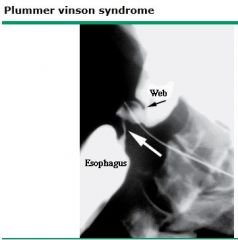
atrophic glossitis, esophageal webs, associated with anemia. Pathophys unknown.
|
benign but associated with development of squamous cell carcinoma of the esophagus.
|
|
|
Treatment of CF
|
Palliative:
1. Give N-Acetylcysteine to loosen mucous plugs 2. Give lots of electrolytes. LOTS! Gene modification therapy is in the works but not yet ready. |
|
|
|
In what age group is suicide most common?
|
Males age 65-74 have the highest rate of suicide in the U.S.
|
|
|
|
What neoplasm(s) are associated with Cirrhosis?
|
Hepatocellular carcinoma
|
|
|
|
List the X-Linked Recessive disorders
|
B W F G O L D H H
(Be Wise, Fool's GOLD Heeds Silly Hopes) Bruton's aggamaglobulinemia Wiskott-Aldrich syndrome Fabry's Disease G6PD Deficiency Ocular Albinism Lesch-Nyhan syndrome Duchenne's muscular dystrophy Hunter's Syndrome Hemophilia A and B |
|
|
|
Define BMI, and its normal ranges.
|
1. kg / m^2|2. Ranges:|18.5 > underweight|18.5 - 24.9 = normal|25 - 29.9 = overweight|30 - 39.9 = Obese|40 < Morbid obesity
|
|
|
|
What neoplasm(s) are associated with Ulcerative Colitis?
|
Colonic adenocarcinoma
|
|
|
|
Duchenne's Muscular dystrophy
Genetics & Biochem |
(1)
X-linked Recessive Deletion/frameshift mutations in Dystrophin protein |
(2) Biochem:
Dystrophin connects Actin of cytoskeleton to sarcolemmal membrane of myocytes. Result of deletion: force of muscle fiber contraction is not transferred to cell membrane; the cell doesn't contract, only its organelle does. |
|
|
What are the brain waves corresponding with each stage of sleep?
|
beta waves: REM & Awake
alpha waves: Awake with eyes closed theta waves: Stage 1 Sleep spindles and bruxism: Stage 2 delta waves: Stage 3 |
|
|
|
What neoplasm(s) are associated with Paget's disease of the bone?
|
1. Secondary osteosarcoma
2. Secondary fibrosarcoma |
|
|
|
Becker's Muscular Dystrophy
Genetics & Biochem |
(1)
X-linked recessive Missense/nonsense Mutation (*NOT* frameshift/deletion) to dystrophin gene. |
(2) Biochem:
Some dystrophin is made, but it's abnormal to a greater or lesser degree. The result is a disorder that's less severe and has a later onset than in Duchenne's. |
|
|
What is the principle neurotransmitter in REM sleep, and where is it acting?
|
ACh is the principle neurotransmitter in this form of sleep.|ACh hyperactivity is seen in the reticular formation.
|
|
|
|
What neoplasm(s) are associated with immunodeficiency states?
|
Malignant lymphomas
|
|
|
|
Muscular dystrophies
Presentation & Diagnostics |
These disorders are inherited and present at anytime from childhood to early adulthood wiht progressive muscle wasting and myocyte degredation.
Histological abnormalities include: Muscle tissue can become replaced with fat. Muscle fibers will vary in size and diameter. |
Diagnose via increased serum Creatinine Kinase and muscle biopsy.
|
|
|
What is the most common pathological sleep symptom in depressed patients?
|
Early waking is the most common sleep symptom in these patients.
|
|
|
|
What neoplasm(s) are associated with AIDS?
|
1. Aggressive malignant lymphomas (non-Hodgkins)
2. Kapoi's sarcoma |
|
|
|
Fragile X
Path & Presentation |
X-linked defect in methylation and expression of the FMRI gene.
Results in trinucleotide expansion repeat of CGG |
Second most common cause of MR!
X-linked, X-tra long gene, Xtra large testes, jaw, and ears!! |
|
|
Activity in what brain center is responsible for eye activity during REM sleep?
|
Hyperactivity in the PPRF is associated with the eye movements characteristic of REM.
|
|
|
|
What neoplasm(s) are associated with autoimmune diseases, i.e. Hashimoto's, thyroiditis, and myasthenia gravis?
|
Lymphoma
Thymomas are common in MG and Lambert-Eaton's (and are a type of lymphoma) |
|
|
|
List the trinucleotide repeat diseases and their repeated sequences.
|
1. Fragile X: CCG
2. Friedrich's ataxia: GAA 3. Huntington's Disease: CAG 4. Myotonic Dystrophy: CTG |
|
|
|
How does depression affect REM sleep?
|
Depression decreases REM latency, while increasing total time spent in REM sleep in these patients. It also increases the amount of time spent in REM early in the sleep cycle.| - *REM latency is the amount of time it takes for the patient to enter REM after falling asleep.
|
|
|
|
What neoplasm(s) are associated with Acanthosis nigricans?
|
Underlying visceral malignancies, i.e. stomach, lung, and uterus.
|
|
|
|
What disorder is often associated with duodenal atresia?
|
Down's Syndrome.
|
|
|
|
Which brain center is responsible for circadian rhythms?
|
The Suprachiasmatic nucleus of the hypothalamus is responsible for this function.
|
|
|
|
What neoplasm(s) are associated with radiation exposure?
(not sun -- i.e. irradiated water) |
Sarcoma
Papillary thyroid cancer |
|
|
|
List the trisomies and their associated syndromes.
|
1. Down's Syndrome: trisomy 21
2. Edward's Syndrome: trisomy 18 3. Patau's Syndrome: trisomy 13 |
Remember:
Down's = Drinking age (21) Edward's = Election age (18) Patau's = Puberty (13) |
|
|
Bulemia symptoms:
|
Eroded tooth enamel
25% present with enlarged parotid glands Irregular menses |
|
|
|
What gene mutation and defective product is associated with CML?
|
ABL ==> BCR-ABL t(9,22) -- The Philadelphia Chromosome translocation!
Together, these genes form a constitutively expressed tyrosine kinase Treatable with imatinib. |
|
|
|
Screening for Down's in Pregnancy
|
1. Decreased α-fetoprotein
2. Increased β-hCG 3. Decreased estriol, 4. normal inhibin A |
|
|
|
What psychiatric complications of childbirth are common, and in what time frames?
|
Maternity Blues: more than half of women, resolves within 10 days.
|
Post-partem depression: 10-20% of women, occurs anywhere from 2 weeks to 1 year post-partem.
|
|
|
What gene mutation is associated with Burkitt's lymphoma?
|
C-MYC mutation on Chromosome 8
Results in constitutive transcription factor expression for HAT's |
|
|
|
Screening for Edward's syndrome in pregnancy
|
1. Decreased α-fetoprotein
2. Decreased β-hCG 3. Decreased estriol 4. Normal inhibin A |
|
|
|
When is it OK to discuss patient information with the patient's spouse? Is it always OK?
|
Only when he is incapacitated.
|
|
|
|
What gene mutation is associated with follicular and undifferentiated lymphomas?
|
BCL-2 mutation
Results in constitutive protection from apoptosis |
|
|
|
Screening for Patau's syndrome in pregnancy
|
1. Normal α-fetoprotein
2. Normal β-hCG 3. Normal estriol 4. Normal inhibin |
|
|
|
What gene mutation is associated with breast, ovarian, and gastric carcinomas?
|
erb-B2 mutation.
Results in constitutive activation of a tyrosine kinase |
|
|
|
Contrast presentations of the three trisomies.
|
1. Down's: flat facies, epicanthal folds, simian crease
2. Edward's: micrognathia, lowset ears, clenched hands 3. Patau's: cleft lip/palate, holoprosencephaly, polydactyly |
|
|
|
What gene mutations are associated with colon carcinoma?
|
1. Ras mutation results in constitutive action of a GTPase, and therefore of a G protein.
2. APC (in FAP) 3. DCC (Deleted in Colon Carcinoma) |
|
|
|
List the chromosomes commonly involved in Robertsonian translocation.
|
Chromosomes 13, 14, 15, 21, 22
Results in loss of short, 'p' arms of chromosomes, and a giant chromosome |
|
|
|
What gene mutation is associated with lung tumors?
|
L-myc mutation
Results in constitutive action of a transcription factor for growth factors. |
|
|
|
Cri-du-chat syndrome
Presentation and Genetics: |
Presentation:
Microcephaly Moderate to severe MR Hi pitched, cat-like scream Congenital VSD |
Congenital microdeletion of short arm of chromosome 5 (46, XX/Xy, 5p-)
|
|
|
What gene mutation is associated with neuroblastoma?
|
N-myc mutation
Results in constitutive action of a transcription factor for growth factors. |
|
|
|
Williams Syndrome
Presentation and Genetics: |
Presentation:
Long, "elfin" facies, MR, hypercalcemia, very friendly and with good verbal skills |
Microdeletion of long arm of chromosome 7. Deleted region includes elastin gene.
|
|
|
What gene mutation is associated with MEN types IIA and IIB? (and papillary thyroid carcinoma?)
|
ret mutation
Results in constitutive action of a tyrosine kinase. |
|
|
|
22q11 deletion of syndromes
Presentation and Genetics: |
Variable presentation including:
Cleft palate Abnormal facies Thymic aplasia Cardiac defects Hypocalcemia CATCH-22 |
Due to microdeletion at 22q11
CATCH-22 |
|
|
What gene mutation is associated with GIST?
|
c-kit mutation
Constitutive activation of a cytokine receptor. Treatable with imatinib |
|
|
|
Vitamin A
3 active forms Generation in body |
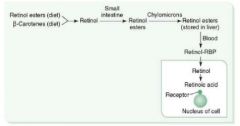
(1)
3 active forms are: retinal retinol retinoic acid |
(2)
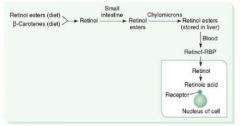
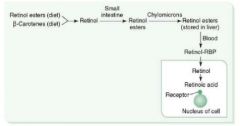
1. Eaten as β-carotenes in green veggies. 2. Esterified in liver to retinol esters. 3. Retinol is secreted from liver and binds to RBP, traveling together to target tissues 4. Retinol is taken up in tissues and irreversibly converted to retinoic acid. 5. Retinoic acid activates gene transcription of protein products. |
|
|
What gene mutation is associated with Retinoblastoma and osteosarcoma?
|
Rb knockout.
Normally, Rb prevents G1-->S cycling |
|
|
|
Physiological Functions of Vitamin A
|
**Antioxidant!
1. Component of visual pigments. 2. Important in normal cell differentiation; prevents squamous metaplasia 3. Bone and tooth development. |
|
|
|
What gene mutation is associated with Li-Fraumeni snydrome?
|
p53 knockout mutation.
p53 is the Guardian of the Genome. It prevents G1-->S cycling. |
|
|
|
Vitamin A
Deficiency & Excess |
Deficiency results in night blindness, dry skin
|
Excess results in:
Arthralgias, fatigue, HA, skin changes, sore throat, alopecia. |
|
|
What gene mutation is associated with Breast and ovarian cancer? What type of gene product is affected?
|
BRCA1 knockout mutation
BRCA1 is a DNA repair protein. |
|
|
|
Di George Syndrome
Presentation & Path: |
Pt. will be an infant sick with chronic infections from birth.
Congenital heart defects, esp tetralogy of Fallot, are commonly associated. Abnormal facies Hypocalcemia d/t absence of parathyroid glands. |
AKA thymic dysplasia, passed on via autosomal dominant inheritance.
Causative mutation results in abnormal development of the 3rd and 4th branchial pouch, so it's often associated with congenital abnormalities and hypoparathyroidism. Diagnosed by severely decreased T cell count. Occurs in 50% of patients with 22q11 deletion syndromes. |
|
|
What gene mutation is associated with breast cancer but not ovarian cancer?
|
BRCA2 knockout mutation.
BRCA2 is a DNA repair protein. |
|
|
|
Velocardiofacial syndrome
Presentation & Path |
Presents with palate, facial, and cardiac defects.
|
Caused by 22q11 mutation. Like DiGeorge's, the branchial arches are affected but unlike DiGeorge's, immunodeficiency is uncommon in these patients.
|
|
|
What gene mutation is associated with melanoma?
|
p16 knockout mutation.
|
|
|
|
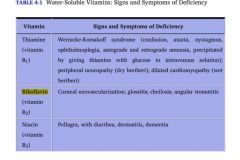
Vitamin B1
MoA |
AKA thiamine or thiamine pyrophosphate (TTP), this nutrient acts as a cofactor in several reactions:
1. Decarboxylation of pyruvate dehydrogenase in glycolysis 2. Decarboxylation of α-ketoglutarate dehydrogenase in the TCA cycle. 3. Transketolase in the HMP shunt 4. Branched-chain AA dehydrogenase. |
|
|
|
What gene mutation is associated with colorectal cancer secondary to FAP?
|
APC gene knockout.
|
|
|
|
Vitamin B1
Deficiency & Excess |
1. Deficiency results in Impaired glucose breakdown and ATP depletion. Highly aerobic tissues like brain and heart are affected first.
2. Wernicke-Korsakoff syndrome 3. Beriberi |
No B1 excess syndromes are known.
|
|
|
What gene mutation is associated with Wilms' tumor?
|
WT1 gene knockout.
**Causes an anaplastic renal sarcoma in kids **Most common renal tumor in kids |
|
|
|
Wernicke-Korsakoff syndrome
Presentation & Path & Etiology: |
Wernicke-Korsakoff:
Classic triad of confusion, ophtalmoplegia, ataxia Also lying, personality change, and memory loss |
Caused by thiamine (B1) deficiency
Absence of cofactor for glycolytic, TCA, and HMP reactions decreases aerobic production of ATP. Highly aerobic tissues are injured first. Common causes of thiamine deficiency are alcoholism and malnutrition. |
|
|
What gene mutation is associated with Neurofibromatosis type 1?
|
NF1 gene knockout
|
|
|
|
Wet vs. Dry beriberi
Presentations Path & Etiologies |
(1)
Wet beriberi: high-output cardiac failure, dilated cardiomyopathy, edema. Hi BP Dry beriberi: polyneuritis, symmetrical muscle wasting |
(2)
Both are caused by thiamine (B1) deficiency. Common causes of B1 deficiency are alcoholism and malnutrition. |
|
|
What gene mutation is associated with neurofibromatosis type 2?
|
NF2 gene knockout
|
|
|
|
Vitamin B2
MoA Deficiency |
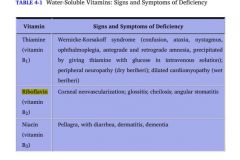
Riboflavin is the precursor to 2 coenzymes in oxidation and reduction reactions: FAD and FMN
|
Deficiency results in:
1. Glossitis Stomatitis Cheilosis (inflammation of lips, scaling and fissures at the corners of the mouth) 2. Corneal vascularization |
|
|
What gene mutation is associated with pancreatic cancer?
|
DPC gene knockout
"Deleted in Pancreatic Cancer" -- think of DCC in colorectal cancer. |
|
|
|
Vitamin B3
Derivatives MoA |
Derived from tryptophan
Synthesis requires B6 |
Constituent of NAD+ and NADP+
**Remember: Niacin is the layman's nickname for Nicotinamide. NAD = Nicotinamide Adenine Diphosphate. Niacin is made into NAD! |
|
|
What gene mutation is associated with colon cancer?
|
DCC gene knockout mutation
(*Deletion causes Colon Cancer) |
|
|
|
Vitamin B3
Deficiency & Excess |
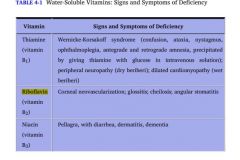
Deficiency: 3 D's of B3
Diarrhea, Dermatitis, and Dementia We call B3 deficiency Pellagra, but it usually specifically refers to the "pearl necklace" of niacin deficiency |
Excess: facial flusing
|
|
|
PSA
|
Prostate-specific antigen.
Elevated in BPH, prostatitis, and most importantly, prostate carcinoma. Used for screening, confirmation, and monitoring for recurrence only. *Not initial diagnosis. |
|
|
|
Deficiencies of Vitamin B2 vs. B3:
|
Riboflavin: 2 C's of B2 (plus stomatitis!)
1. Cheilosis (inflammation and scaling of the lips, stomatitis & glossitis) 2. Corneal vascularization |
Niacin: 3 D's of B3
Diarrhea Dermatitis Dementia |
|
|
Prostatic acid phosphatase
|
Marker for prostate carcinoma
|
|
|
|
Vitamin B5
(1) MoA (2) Deficiency |
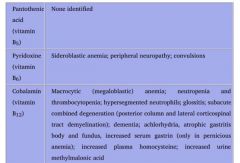
Pantothenate
Essential component of: 1. CoA 2. Fatty acid synthase **We can't make anything without it! |
Deficiency uncommon.
Dermatitis Enteritis Alopecia Adrenal insufficiency **Rapidly-dividing epithelial cells are injured first! |
|
|
CEA
|
Carcinoembryonic antigen.
Marker found in ~70% of: 1. Colorectal cancers 2. Pancreatic cancers. Non-specific b/c also found in gastric, breast, and thyroid medullary cancers. |
|
|
|
Vitamin B6
MoA What do we need it to make? |
Pyridoxine
Converted to a cofactor in: 1. Transaminations (AST & ALT) 2. Decarboxylation reactions 3. Glycogenolysis (glycogen phosphorylase) |
We need pyridoxine to make:
1. Histamine 2. Pyruvate from serine in gluconeogenesis 3. Niacin from tryptophan 4. Heme |
|
|
α-fetoprotein
|
Normally found in fetus. Elevated in:
1. Hepatocellular carcinomas 2. Yolk Sac Tumor |
|
|
|
Hartnup disease
|
Decreased intestinal absorption and renal reabsorption of neutral amino acids.
Results in low tryptophan Excess tryptophan is normally converted into niacin, therefore low tryptophan = low niacin Pellagra and B3 deficiency result. |
|
|
|
β-hCG
|
Elevated in:
1. Chorionic carcinoma (non-seminomatous germ cell tumor) 2. Mole pregnancy |
|
|
Causes of B3 deficiency:
|
Deficiency caused by:
1. Malnutrition 2. Hartnup's disease: inability to absorb B3 in intestine and to reabsorb it in urine. 3. Carcinoid Syndrome: b/c its precursor, tryptophan, is instead being made into serotonin! |
|
|
|
CA-125
|
Elevated in malignancies of the ovarian surface epithelium
|
|
|
Vitamin B6 deficiency
Effects: Causes |
1. Sideroblastic anemia
2. Convulsions 3. Peripheral neuropathy |
Deficiency can be caused by:
1. Isoniazid 2. Unfortified goat milk. |
|
|
S-100
|
Elevated in:
1. Melanoma 2. Neural tumors 3. Astrocytomas |
Highly sensitive for melanocyte dedifferentiation indicating meloma
Also produced by neural crest-derived cells, so it'll be elevated in neural/glial tumors. |
|
|
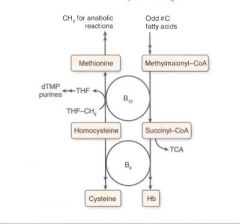
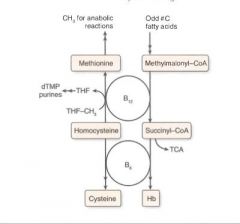
B12
(1) MoA (2) Deficiency |
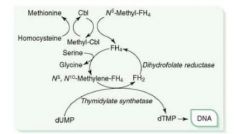
Accepts methyl groups from both N5-methyl-TH4 and homocysteine, activating TH4 and deactivating homocysteine!
Therefore, it is necessary for: 1. reducing serum levels of homocysteine, which helps prevent arterial disease. 2. Methylation of dUMP to dTMP. |
Cobalamin deficiency results in:
1. Macrocytic, megaloblastic anemia d/t dTTP shortage 2. Atherosclerotic disease d/t homocysteinemia 3. Neurologic symptoms d/t abnormal myelin |
|
|
Alkaline phosphatase
(bone variant) |
Elevated in:
1. Mets to bone 2. Obstructive biliary disease 3. Paget's disease of bone |
Alk-phos is made by the liver. It is also made by and tethered to osteoblasts.
Alk-phos is therefore a good indicator of osteoblastic activity. If elevated, consider: 1. osteoblastic-osteoclastic stage of Paget's disease 2. osteoblastic bone mets |
|
|
Where do we get our B12?
|
We get it from animal products, but it is only made by microorganisms.
Most people have a several year storage pool in the liver. |
|
|
|
Pt. presents with elevated serum Bombesin.
DDx: |
Elevated in:
1. Neuroblastoma 2. Lung cancer 3. Gastric cancer |
AKA GRP (Gastrin-Releasing Peptide), Bombesin is a GI neurocrine, made by the neurons of the gastric mucosa. It will be increased in gastric hyperplasia/tumor.
|
|
|
Causes of B12 Deficiency:
|
1. Malabsorption, i.e.:
Celiac sprue Enteritis Diphyllobothrium latum 2. Lack of intrinsic factor, i.e.: pernicious anenia gastric bypass absence of terminal ileum in Crohn's |
|
|
|
TRAP
|
Tartrate-resistant acid phosphatase
Elevated in: *Hairy* cell leukemia, the pre-plasma cell, B cell malignancy Catch that "Hairy" animal in the "TRAP" |
|
|
|
Distinguishing folic acid deficiency from B12 deficiency
|
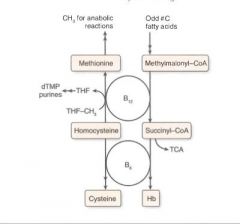
The syndromes are distinct in that there are no neurological symptoms in folic acid deficiency, but many in B12 deficiency.
|
|
|
|
CA-19-9
|
Elevated in pancreatic adenocarcinoma
|
|
|
|
Causes of folate deficiency:
|
Most common vitamin deficiency in the U.S.
Usually caused by pregnancy or alcoholism. Malabsorption disorders Tapeworm Chronic pancreatitis Drugs: Phenytoin Sulfonamides MTX |
|
|
|
Calcitonin
|
Elevated in thyroid medullary carcinoma
|
|
|
|
Biotin
(1) MoA (2) Deficiency |
This nutrient is a cofactor for carboxylation reactions.
Cofactor in the following reactions: 1. Pyruvate carboxylase 2. A.CoA carboxylase 3. Propionyl CoA carboxylase |
Dermatitis
Alopecia Enteritis Adrenal Insufficiency **Same as B5, which, not incidentally, also is involved in A.CoA synthesis! |
|
|
Which neoplasms may secrete ACTH?
|
Small cell carcinoma may secrete ACTH or ACTH-like peptide
Result: Cushing's syndrome |
|
|
|
3 Functions of Vitamin C
|
1. Hydroxylation of Collagen
deficiency = scurvy] 2. Absorption of iron (by reducing it in the gut; Fe3+ can't be absorbed) [deficiency = microcytic anemia] 3. Necessary for DA beta-hydroxylase to convert DA to NE. [depression] |
|
|
|
Which neoplasms may secrete ADH?
|
SIADH, and hypervolumemia may result from ADH secretion by:
1. Small cell lung carcinomas 2. Intracranial neoplasms |
|
|
|
In what forms do we take in vitamin D from (1) plants and (2) milk?
What other forms exist in human physiology? |
D2 = ergocalciferol, which is ingested from plants
D3 = cholecalciferol |
Monohydroxy D3 (25-OH D3): storage form of calciferol
Dihydroxy D3 (1,25-OH D3): active form. |
|
|
There are 3 neoplasms that may secrete either PTH-related peptide, TGF-β, TNF-α, or IL-1, and are known for causing *humoral hypercalcemia of malignancy*. List them.
|
While some malignancies cause hypercalcemia because they are directly osteolytic, others are associated with humoral hypercalcemia of malignancy. These include:
1. Squamous cell cancer of the lung 2. Renal cell carcinoma 3. Breast carcinoma |
|
|
|
Distinguish mechanisms for conversion to active Vitamin D in endogenous vs. ingested Vitamin D.
|
Endogenous:
D3 --> 25-OH D3 (liver) --> 1,25-OH D3 (kidney) |
Exogenous:
dehydrocholesterol --> Vitamin D3 (photoconversion in skin) --> 25-OH D3 (liver) --> 1,25-OH D3 (kidney) |
|
|
Which neoplasm may cause erythropoietin oversecretion?
Which neoplasms may result? |
Tumors that cause elevated erythropoietin:
1) Renal cell carcinoma 2) Hepatocellular carcinoma **Both are derived from cells that normally make EPO |
Elevated erythropoietin, resulting in polycythemia, may be associated with (though not necessarily caused by):
1. Hemangioblastoma 2. Pheochromocytoma |
|
|
Vitamin E
Function & Deficiency |
Function: Anti-oxidant
|
Deficiency:
1. Hemolytic anemia d/t radical damage |
|
|
(1) Which neoplasm may secrete antibodies against Ca2+ channels at the neuromuscular junction?
(2) Presentation |
Anti-Ca2+ channel antibodies are sometimes secreted by:
1. Small cell lung carcinoma (most common cause) 2. Thymoma |
Lambert-Eaton Syndrome:
1. Weakness that improves as the day goes on 2. Most pronounced in the proximal muscles. |
|
|
Vitamin K
MoA: Deficiency: |
Gamma-Carboxylation, i.e. of the coagulation factors!
|
Increased PT/INR *and* aPTT!!
|
|
|
Excess nucleic acid turnover (and subsequent gout and urate nephropathies) may result from which kinds of neoplasms?
|
Leukemias and lymphomas.
|
|
|
|
Vitamin K is necessary for the synthesis of which proteins in the coagulation cascade?
|
This vitamin is necessary for synthesis of
Clotting factors: 2, 7, 9, 10 Clotting cofactors: Protein C and Protein S |
|
|
|
Paraneoplastic causes of Cushing's Syndrome:
|
1. Small cell lung carcinoma
2. Pancreatic carcinoma 3. Neural tumors *All 3 are causing a Cushing's Disease by secreting ACTH |
|
|
|
Zinc deficiency:
|
Deficiency of this nutrient results in delayed wound healing, hypogonadism, decreased growth of adult hair, anosmia and dysgeusia.
|
|
|
|
Paraneoplastic causes of inappropriate antidiuretic secretion:
|
1. Small-cell carcinoma of the lung
2. Intracranial neoplasms May secrete either ADH or natriuretic hormones. |
|
|
|
Which deficiency/excess of which nutrient causes anosmia and dysgeusia?
|
Zinc deficiency causes anosmia (lack of smell) and dysgeusia (aberrant sense of taste -- i.e. metallic taste on back of mouth)
|
|
|
|
Paraneoplastic causes of Hypercalcemia:
|
1. Squamous cell carcinoma of the lung
2. Breast carcinoma 3. Renal carcinoma 4. Adult T-cell leukemia/lymphoma **These tumors can secrete either PTHRP, TGF-alpha, TNF, or IL-1. |
|
|
|
Mechanism of ethanol metabolism:
|
Ethanol --ethanol dehydrogenase--> acetaldehyde --acetaldehyde dehydrogenase--> Acetate
|
|
|
|
Paraneoplastic causes of hypoglycemia:
|
1. Ovarian carcinoma
2. Fibrocarcinoma (breast cancer) 3. Other mesenchymal sarcomas **These tumors secrete insulin or insulin-like substance. |
|
|
|
Disulfiram
MoA: |
Inhibits acetaldehyde dehydrogenase, increasing acetaldehyde buildup.
Acetaldehyde causes hangover symptoms! |
**This drug induces hangover by interrupting the last phase of ethanol breakdown to acetate!
|
|
|
Paraneoplastic causes of carcinoid syndrome:
|
1. Hepatocellular carcinoma
2. Bronchial adenoma 3. Pancreatic carcinoma **These tumors secrete Serotonin or bradykinin. |
|
|
|
Fomepizole
MoA: |
Inhibits alcohol dehydrogenase and is an antidote for methanol or ethylene glycol poisoning.
|
|
|
|
Paraneoplastic causes of polycythemia:
|
1. Renal cell carcinoma
2. Hepatocellular carcinoma **Both secrete EPO |
|
|
|
How does ethanol consumption alter normal glucose metabolism?
|
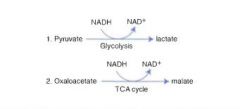
Ethanol metabolism causes NADH to build up in the liver. To restore NAD+, we have to reverse 2 major pathways:
Pyruvate ---> Lactate instead of A.CoA Oxaloacetate ---> malate instead of citrate! |
Result:
1. Depletion of pyruvate to make new NAD+ results in decreased gluconeogenesis ---> hypoglycemia! 2. Downregulation of TCA d/t depletion of oxaloacetate |
|
|
Paraneoplastic causes of myasthenia:
|
**1. Thymoma/thymic hyperplasia
2. Small cell Lung cancer **It's believed that MG is caused by B cells in the thymus secreting anti-AChR antibodies!! |
|
|
|
Define marasmus
|
Energy malnutrition resulting in tissue and muscle wasting, loss of subcutaenous fat, and variable edema.
|
|
|
|
Paraneoplastic causes of disorders of the central and peripheral nervous system
|
Breast carcinoma
|
|
|
|
What metabolic processes occur in the mitochondria?
What processes occur in the cytoplasm? |
1. TCA
2. ETC 3. Fatty acid oxidation 4. A.CoA production |
1. Glycolysis
2. Fatty acid synthesis 3. HMP Shunt 4. Protein synthesis (RER) 5. Steroid synthesis (SER) |
|
|
Rate-limiting step of Glycolysis
|
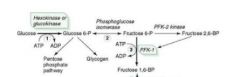
Fructose-6-phosphate --PFK-1--> Fructose-1,6-phosphate
|
|
|
|
Rate-limiting step of Gluconeogenesis:
|
Fructose-1,6-bisphosphonate
|
|
|
|
Rate-limiting step of TCA cycle:
|
Isocitrate dehydrogenase
|
|
|
|
Rate-limiting step of glycogen synthesis
|
Glycogen synthase
|
|
|
|
Rate-limiting step of glycogenolysis
|
Glycogen phosphorylase
|
|
|
|
Rate-limiting step of HMP shunt
|
G6PD
|
|
|
|
Rate-limiting step of de novo pyrimidine synthesis:
|
Carbamoyl phosphate synthetase II
|
|
|
|
Rate-limiting step of de novo purine synthesis:
|
Glutamine-PRPP amidotransferase
|
|
|
|
Rate-limiting step of Urea cycle
|
Carbamoyl phosphate synthetase I
|
|
|
|
Rate-limiting step of Fatty acid synthesis
|
Acetyl-CoA carboxylase
|
|
|
|
Rate-limiting step of Fatty acid oxidation
|
Carnitine acyltransferase I
|
|
|
|
What's the difference between the actions of biotin and folic acid?
|
Biotin is a cofactor in carboxylation reactions -- it adds CO2
THF is methyl group donor -- it adds CH3 |
|
|
|
What is the difference between the activity of NADH and NADPH?
|
NADH is usually involved in catabolic processes, i.e. Glycolysis and the TCA
NADPH is usually involved in anabolic processes, like steroid and fatty acid synthesis. |
|
|
|
HMP Shunt:
(1) What does it do? (2) Describe the most prominent associated pathology. |
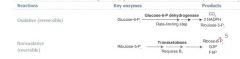
NADPH is made by the HMP shunt, AKA the pentose-5-phosphate pathway.
A small fraction of the Glucose-6P that's made in the first step of glycolysis gets shunted off to this pathway to restore NADP+ to NADPH. |
G6PD is the rate-limiting step of the shunt. When it is defective, NADPH deficiency occurs which is minor in most tissues, but not in the erythrocytes, where the shunt is the only source of NADPH.
Result: oxidative/radical damage to RBCs and hemolytic anemia. |
|
|
How do hexokinase and glucokinase differ?
(1) General description (2) Kinetics |
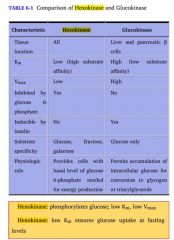
Both of these enzymes phosphorylate glucose as part of the first step of glycolysis.
Hexokinase is found in all cells and specializes in operating in a low glucose environment to provide a small amount of glucose for each cell's needs. Glucokinase is found in the liver, and specializes in working in a hi glucose environment to rapidly phosphorylate lots of glucose, providing glycogen storage for the whole body. and is inhibited by the presence of glucose 6-P. This allows individual cells to burn only as much glucose as they need. Glucokinase is found in the liver, and is not; this allows the organ to function as a glycolytic machine for the rest of the body. |
Hexokinase has a hi affinity for glucose and a LOW K_m, so it can operate in low glucose environments.
It also has a low V_m, so that regardless of the environment, it doesn't make a lot Glucokinase has a LOW substrate affinity and a HI K_m, but a very hi V_max, so it works best where there's lots of glucose, and makes stuff for the whole body. |
|
|
(1) What is the rate-limiting step of glycolysis, and (2) how is it regulated?
|

Fructose-6P + ATP --PFK-1--> Fructose-1,6-bisphosphate
|
As the rate-limiting enzyme, PFK-1 is:
Downregulated by ATP, Citrate Upregulated by AMP, fructose-2,6-BP |
|
|
Which two steps of glycolysis consume ATP?
|

Glucose --Gluco/Hexokinase--> G-6P
Fructose-6P --PFK-1--> Fructose-1,6-bisphosphate |
|
|
|
In which two steps does glycolysis produce ATP?
|
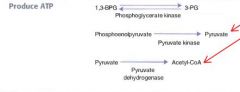
#1: yields 2 ATP
1,3-bisphosphoglycerate --phosphoglycerate kinase--> 3-phosphoglycerate #2: also yields 2 ATP Phosphoenolpyruvate--Pyruvate kinase--> Pyruvate |
|
|
|
How do blood sugar levels interact with the rate-limiting step of glycolysis?
(main idea) |
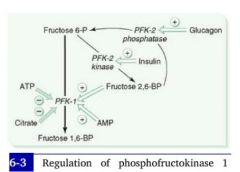
Background:
2 enzymes act on the substrate of hte rate-limiting reaction: PFK-1 and PFK-2 PFK-1 is responsible for the main substrate. **In the presence of insulin, PFK-2 is activated. **PFK2's activity effectively induces PFK-1 (and therefore glycolysis). |
|
|
|
(1) What is the mechanism by which insulin interacts with the rate of glycolysis?
(2) How about glucagon? |
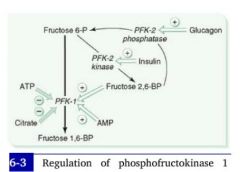
Hi blood sugar --> hi insulin --> decreased cAMP --> dephosphorylation of PFK-2 gives it a kinase activity --> increased production of Fructose-2,6-bisphospate --> increased PFK activity --> increased glycolysis.
|
Low blood sugar --> hi glucagon --> hi cAMP in cells --> PFK-2 is phosphorylated and given a phosphatase activity --> PFK-2 reconverts Fructose-2,6-bisphosphate to fructose-6P --> Fructose-2,6-Bisphosphate levels decrease and PFK-1 is downregulated --> Glycolysis stops.
|
|
|
What cofactors are required for pyruvate processing enzymes to function?
|

1. Pyrophosphate (Thamine/TTP)
2. FAD (B2, riboflavin) 3. NAD (B3, niacin) 4. CoA (B5, pantothenate) 5. Lipoic Acid |
We treat the enzyme complex responsible for pyruvate processing as though it were a single enzyme, pyruvate dehydrogenase, but obviously it's 3 enzymes. Don't worry about them.
|
|
|
Pyruvate Dehydrogenase Deficiency
Etiology & Presentation |

Causes:
1. Genetic deficiency 2. Cofactor deficiency (i.e. B1 def in alcoholism) |
Presentation:
Neurological defects |
|
|
So we've made pyruvate from glucose. What are the 4 things we can do with it?
|

Can be made into:
1. A.Coa for the TCA 2. Lactic acid fermentation 3. Oxaloacetate for either Gluconeogenesis or TCA 4. We can package waste NH3 from the periphery into it, turning it into alanine. [This is then shipped back to the liver for entry into the Urea Cycle.] |
|
|
|
What are the products of one turn of the TCA Cycle?
|
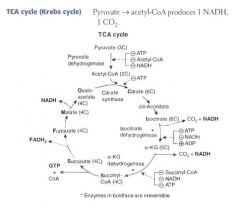
For each turn of the cycle, we burn 1 A.CoA. The net output is:
3 NADH 1 FADH2 1 GTP 2 CO2 = 12 ATP |
|
|
|
What are the enzymes of the TCA cycle?
|
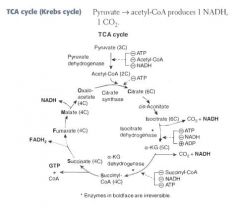
Citrate Is Krebs Starting Substrate For Making Oxaloacetate!!
Citrate Isocitrate α-ketoglutarate Succinyl CoA Succinate Fumarate Malate Oxaloacetate |
|
|
|
Where and how is α-ketoglutarate metabolized?
|
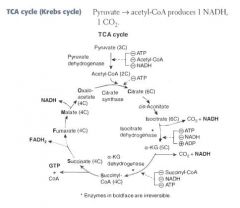
α-ketoglutarate is a metabolite of the TCA cycle:
α-KG --α-KG dehydrogenase--> Succinyl CoA |
α-ketoglutarate dehydrogenase is interesting because it is just like pyruvate dehydrogenase!
It's a complex of 3 enzymes with the same 5 cofactors: B1, B2, B3, B5, lipoic acid. |
|
|
How does arsenic affect cellular metabolism?
|

Arsenic inhibits lipoic acid from acting as a cofactor in pyruvate dehydrogenase.
The result is neurological problems, lactic acidosis |
|
|
|
Arsenic poisoning
Presentation: |
This type of toxicity presents with vomitting, rice-water stools, and garlic breath.
|
|
|
|
How does NADH made in the cytoplasm during glycolysis get restored to NAD+, so glycolysis can continue?
|
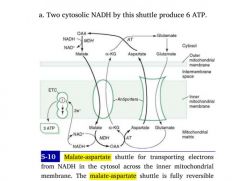
NAD+ and NADH CANNOT cross the inner mitochondrial membrane!
Instead, oxaloacetate is converted to a non-TCA substrate -- aspartate -- which crosses through a channel. |
1. Oxaloacetate --> Aspartate
2. Aspartate crosses opposite glutamine 3. Aspartate is reconverted to oxaloacetate in the cytoplasm 4. OAA accepts H:- from NADH, becoming malate 5. Malate crosses back into matrix to be reused by TCA |
|
|
List the basic amino acids.
|
Lysine
Arginine Histidine (all positively charged in serum) |
|
|
|
List the acidic amino acids.
|
Aspartate
Glutamate (both negatively charged in serum) |
|
|
|
Histone Structure
|
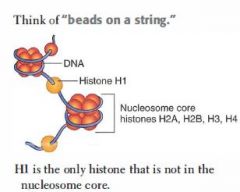
Tetramers of H2A, H2B, H3, and H4 proteins form nucleosome core.
DNA wraps twice around each core. H1 proteins are not in the nucleosome core, but rather tie the nucleosome beads together. |
|
|
|
What are the two functions of methylation DNA methylation?
|

(1) Template strand cytosine and adenine residues are methylated, indicating to DNA polymerase which strand is the template for repairs.
(2) Hypermethylation: CpG islands of DNA tend to become hypermethylated, inactivating transcription. -- CpG islands are associated with the promoters for housekeeping genes that we want to express at low basal rates. |
|
|
|
Role of acetylation in gene expression.
|
This modification, performed by histone acetyltransferases (HATs), loosens DNA from heterochromatin to euchromatin, increasing transcription.
|
|
|
|
Distinct characteristics of the 5 nucleotides:
|
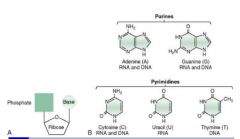
Cytosine: ammonia group
Uracil: ketone Thymine: methylated Adenine: ammonia group Guanine: ketone + ammonia group |
|
|
|
What simple pyrimidine modification is frequently responsible for mutations, and how is it repaired?
|
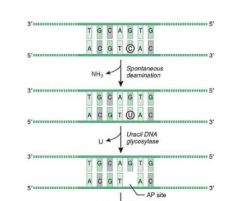
Cytosine deaminates to uracil spontaneously and at a constant rate.
-- C ==> U, resulting in an AT mismatch -- Repaired by BER |
|
|
|
How do healthy cells combat skin cancers?
|
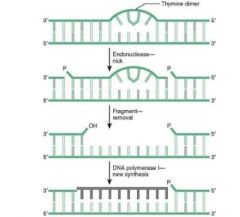
This mutation is combated with Nucleotide Excision Repair.
1. In NER, excision endonuclease detects the dimer b/c the shape is distorted, and nicks the damaged strand on both sides of the lesion, perhaps several bases down. 2. Exonucleases remove the fragment 3. DNA Polymerase I replaces the missing fragment, and DNA Ligase ligates it into the strand. |
Thymidine dimer formation is the most common cause of skin cancers, i.e.:
-- Melanoma -- Basal cell carcinoma -- Squamous cell carcinoma |
|
|
What simple purine modification is responsible for mutations, and how does it happen?
|
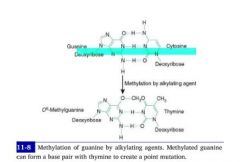
Methylation of Guanine by alkylating agents, i.e. cytoxan, results in it's mismatch with thymine.
-- G-->C -- met-G-->T |
|
|
|
State Mechanism of de novo purine synthesis. Note requirements for production of both bases.
|
1. Ribose 5-phosphate --PRPP Synthetase--> PRPP
2. PRPP + NH3 from glutamine ---> 5-Phosphoribosylamine -->-->--> Inosine monophosphate (IMP) 3a. IMP + aspartate & GTP ---> AMP 3b. IMP + NAD & ATP --> GMP |
**Guanosine requires ATP and NAD+ for its generation.
**Adenosine requires GTP for its generation. |
|
|
What is the committed step of de novo purine synthesis?
|
PRPP + NH3 from glutamate ---> 5-ribosylamine + PPi
|
|
|
|
What is the rate-limiting step in de novo purine synthesis?
|
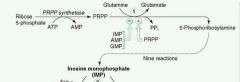
Ribose-5-phosphate ---PRPP Synthetase--> PRPP
|
|
|
|
What is the major difference between purine and pyrimidine synthesis?
|
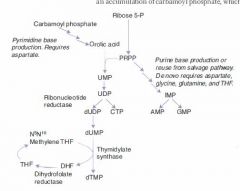
In purine synthesis we start with a ribose backbone and build a base to it.
-- *Inosine* In pyrimidine synthesis we make a temporary base and add the backbone to it, then modify the base. -- **Orotic Acid** |
|
|
|
What is the major difference between purine and pyrimidine synthesis?
|
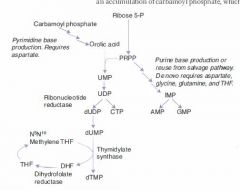
In purine synthesis we start with a ribose backbone and build a base to it.
-- *Inosine* In pyrimidine synthesis we make a temporary base and add the backbone to it, then modify the base. -- **Orotic Acid** |
|
|
|
Hydroxyurea
MoA & Indications |
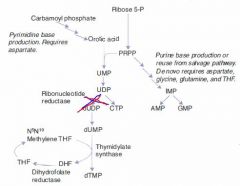
Inhibits thymine production by inhibiting ribonucleotide reductase
|
Indications:
Chemotherapeutic agent used to keep white count low in AML. |
|
|
Hydroxyurea
MoA & Indications |
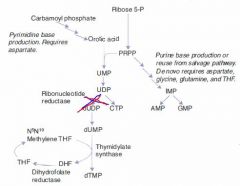
Inhibits thymine production by inhibiting ribonucleotide reductase
|
Indications:
Chemotherapeutic agent used to keep white count low in AML. |
|
|
6-mercaptopurine
MoA and Ind |
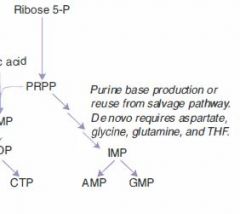
A false purine analogue that both:
(1) inhibits production of real purines via feedback inhibition (2) gets incorporated into DNA, terminating DNA synthesis. |
Maintenance therapy for children with ALL.
Often given as azothioprine, a precursor. |
|
|
6-mercaptopurine
MoA and Ind |
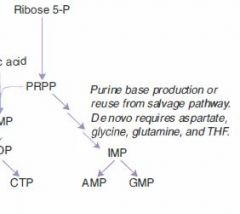
A false purine analogue that both:
(1) inhibits production of real purines via feedback inhibition (2) gets incorporated into DNA, terminating DNA synthesis. |
Maintenance therapy for children with ALL.
Often given as azothioprine, a precursor. |
|
|
5-fluorouracil
MoA & Ind |
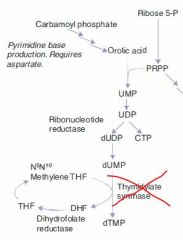
Tumor cells convert it to a false fdUMP, which irreversibly inhibits thymidylate synthase.
This blocks thymidine synthesis, resulting in a thymidine-less death. |
|
|
|
Mechanism of Pyrimidine Synthesis:
|
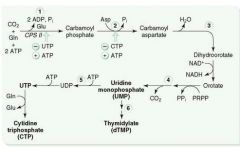
1a. As with purine synthesis, we make PRPP from Ribose-5-phosphate.
1b. CO2 + Gln + ATP ---CPT II---> Carbamoyl Phosphate 2. Carbamoyl Phosphate -->-->--> Orotic Acid 3. Orotic Acid + PRPP ---> UMP 4a. UMP ---> dUMP (or fdUMP in chemo) 4b. UMP ---ribonucleotide reductase---> CTP 5a. dUMP ---Thymidylate synthase + met-THF---> dTMP |
|
|
|
Causes of orotic aciduria.
|
(1) Inability to convert orotic acid to UMP caused by autosomal recessive deficiency in either:
-- orotic acid phosphoribosyltransferase -- orotidine 5'-phosphate decarboxylase |
|
|
|
Presentation of orotic aciduria
|
Symptoms seen in infancy:
1. Increased orotic acid in urine 2. Megaloblastic anemia 3. Failure to thrive |
|
|
|
Clinical findings in orotic aciduria:
|
Symptoms seen in infancy:
1. Increased orotic acid in urine 2. Megaloblastic anemia 3. Failure to thrive |
|
|
|
What is the committed step of pyrimidine synthesis?
|
Gln + CO2 + 2ATP ---CPT II---> Carbamoyl phosphate
The production of carbamoyl phosphate is the committed and rate-limiting step of pyrimidine synthesis. |
|
|
|
2 Purine Breakdown Pathways:
|
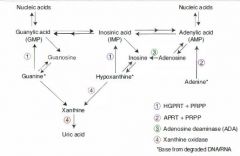
(1)
1. AMP ---AMP deaminase---> IMP 2. IMP ---> Inosine 3. Inosine ---> Hypoxanthine 4. Hypoxanthine ---Xanthine oxidase---> Xanthine 5. Xanthine ---Xanthine oxidase---> Uric Acid (2) 1. AMP ---> Adenosine 2. Adenosine ---Adenosine Deaminase---> Inosine 3, 4, 5: same as above |
|
|
|
SCID
Path & Presentation: |
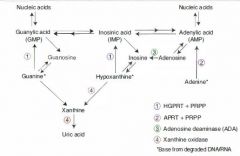
Adenosine deaminase (ADA) deficiency results in:
1) Buildup of ATP b/c we can't break it down. ATP buildup downregulates ribonucleotide reductase, resulting in decreased DNA synthesis. 2) inability to break down adenosine into inosine. Buildup of adenosine is toxic to B and T lymphocytes. |
Presentation: Bubble boy
|
|
|
Lesch-Nyhan syndrome
Path & Presentation: |
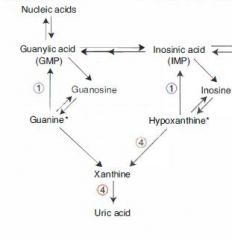
Path:
Caused by X-linked Recessive deficiency of HGPRT, which converts both hypoxanthine and guanine to IMP. Results: (1) Hypoxanthine buildup ---> excess uric acid production (2) Guanine buildup ---> excess uric acid production. |
Presentation:
MR, aggression, self-mutilation, choreoathetosis, hyperuricemia and gout. |
|
|
DNA Polymerase I
|
Prokaryotic only. Degrades RNA primer and fills gaps.
|
|
|
|
DNA Polymerase III
|
*Prokaryotes only*. Elongates leading strand by adding deoxynucleotides until it reaches primer of preceding fragment, then falls off.
|
|
|
|
What pathology results from loss of nonhomologous end joining?
|
Ataxia telangiectasia: disease consisting of cerebellar ataxia, dilation of blood vessels in skin and eyes, and immunodeficiency of T and B cell origin.
|
|
|
|
Which carbon on an incoming nucleotide bears the triphosphate?
|
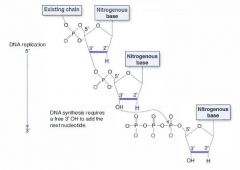
The 5' carbon bears the triphosphate.
The 3' carbon has the hydroxyl group on which the next nucleotide is attached. |
|
|
|
Stop and Start Codons:
|
Start: AUG (codes for methionine)
End: UGA U Go Away UAA U Are Away UAG U Are Gone |
|
|
|
What is the role of promoters and enhancers?
|
Promoters are sequences of DNA upstream from that are bound by RNA Polymerase and other TF's.
Enhancers are DNA sequences that bind TFs to enhance transcription. *They may be upstream, downstream, or IN an INTRON of the gene they enhance!* |
|
|
|
What enzyme is responsible for transcribing:
Eukaryotic rRNA? Eukaryotic mRNA? Eukaryotic tRNA? |
rRNA: RNA Polymerase I
mRNA: RNA Polymerase II tRNA: RNA Polymerase III |
|
|
|
List and describe the various permutations of RNA, IN ORDER, en route to the mature transcript.
|
1) Heterogenous nuclear RNA (hnNRA)
-- completely unprocessed. -- if it's destined for translation, it's called pre-mRNA 2) mRNA -- transcript that's been capped, tailed, and spliced *IN THE NUCLEUS* |
|
|
|
What is the polyadenylation signal?
|
AAUAA
This RNA sequence signals that for the poly-A tail to be attached here by poly-A polymerase. |
|
|
|
What is the polyadenylation signal?
|
AAUAAA
This RNA sequence signals that for the poly-A tail to be attached here by poly-A polymerase. |
|
|
|
Splice mechanism, quick and dirty:
|
1. snRNP's bind primary transcript at splicing sites (marked at either end by GU and AG sequences)
2. snRNP's form a spliceosome, there, and fold the intron into a lariat (loop) 3. Splice! |
snRNP's = small nuclear ribonucleoproteins.
|
|
|
How does lupus interfere with production of mature mRNA?
|
Patients with lupus often make antibodies to spliceosomal snRNP's, disrupting splicing.
|
|
|
|
How do the presence of glucose and lactose affect gene transcription in e. coli?
|
This sugar binds a repressor protein, interfering with its constitutive binding to the promoter sequence of the lac operon.
|
The presence of this sugar inhibits the activator of the lac operon, the CAP protein, from binding to the lac operon promoter.
It does so indirectly, by downregulating cAMP presence in the cell, which is necessary for CAP's activating behavior. |
|
|
What sequence is at the 3' end of tRNA, and why does this matter?
|
CCA: found at the 3' end of tRNA. The amino acid binds at this end.
CCA: Can Carry Amino acids |
|
|
|
What drug interferes with the interaction between amino-acyl tRNA and the 30S ribosomal subunit?
|
Tetracyclines attack these proteins specific to bacteria.
|
|
|
|
List the drugs that bind the 30S subunit of bacterial ribosomes to prevent mRNA translation. How do they do so?
|
1) Aminoglycosides bind 30S ribosomal subunit, preventing initiation.
2) Tetracyclines bind the 30S ribosomal subunit, blocking access of charged tRNA's to the ribosomal subunit. |
Aminoglycosides: Streptomycin, Amikacin
Tetracyclines: demeclocycline, doxycycline |
|
|
(1) List the toxins that interfere with protein translation in humans. (2) How do they do so?
|
1. Shiga toxin
2. Shiga-like toxin (made by EHEC) 3. Diphtheria toxin |
1. This toxin behaves just like tetracycline but in eukaryotes, preventing the binding of charged tRNA to the ribosomal complex.
2. Almost the same toxin is made by enterohemorrhagic E. coli. 3. This toxin inactivates EF-2, preventing the translocation step of protein translation in eukaryotes; the ribosome cannot move down the transcript. |
|
|
What are the major proteins associated with the major steps of protein translation?
|
1. Initiation: IF's
2. Elongation & Translocation: EF's 3. Termination: RF's (release factors) |
|
|
|
Distinguish Translocation and Elongation in protein translation.
|
Elongation refers to the new peptide bond that's formed.
Translocation refers to the movement of the ribosome down the mRNA transcript to the next codon (in the 3' direction) |
|
|
Ribosomal binding sites:
|
A site: Activation site, where aminoacyl-tRNA first bind
P: site of Peptide elongation E: Exit site, site of termination APE |
|
|
|
List the drugs that bind the 50S ribosomal subunit.
|
Macrolides bind the 50S subunit, preventing ribosomal translocation and halting protein translation.
Clindamycin does the same thing. Chloramphenicol binds the 50S subunit, but it prevents *elongation. |
Macrolides: erythromycin, azithromycin, etc.
Chloramphenicol and Clinda are synthetic antibiotics, and are in their own classes. Clindamycin causes C diff Chloramphenicol is cheap! |
|
|
Biochemical role of Mannose-6-phosphate
|
This sugar is added to lysozomal enzymes while still in the Golgi apparatus, marking them for shipment to the lysozomes.
|
|
|
|
List the main vesicular coating compounds and their corresponding destinations.
|
1. COPI: Retrograde transport from Golgi back to ER
2. COPII: anterograde transport from ER to cis-Golgi 3. Clathrin: transport from trans-Golgi to surface membrane |
|
|
|
I-cell Disease / Mucolipidosis II
Path & Presentation: |
Lysozomal storage disorder in which inability to add mannose-6-phosphate to lysozomal enzymes results in their being secreted into the extracellular space.
This lysozomal storage disorder looks like MPS but is characterized by inability to transport many different lysozomal enzymes rather than an inability to make a certain lysozomal enzyme. |
Presentation: coarse facial features, clouded corneas, restricted joint movement, hi plasma levels of lysozomal enzymes. Often fatal in childhood.
|
|
|
List the 5 drugs that act on microtubules.
|
1. Mebendazole/thiabendazole
(antihelminic) 2. Griseofulvin (antifungal) 3. Vincristine/vinblastine 4. Paclitaxel (anti-breast cancer) 5. Colchicine (anti-gout) |
|
|
|
Kartagener's Syndrome
Path & Presentation |
Immotile cilia d/t a dynein arm defect.
(dynein arm |
Infertility, sinusitis, bronchiectases
Also associated with situs inversus |
|
|
How do cilia move?
|
Made of microtubules with dynein and kinesin arms attaching each to a neighboring microtubule.
Dynein: walks away from the base of the cilia (retrograde) Kinesin: walks toward center of cilia (anterograde). |
|
|
|
Chediak-Higashi syndrome
Path & Presentation |
Recessive mutation to MAP proteins.
MAP proteins stabilize microtubules, preventing "catastrophe". Simply put, its a defect in microtubular polymerization that results in decreased organelle and granule transportation, especially in: Neutrophils Lymphocytes Melanocytes |
A child presents with:
1. oculocutaneous albinism (white hair, red eyes) 2. frequent pyogenic infections. |
|
|
Describe cilia's biochem.
|
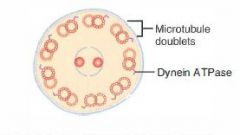
9 + 2 microtubule arrangement
9 microtubule doublets form a circle around a central doublet. Axonemal Dynein links the peripheral doublets together so they move in concert. |
|
|
|
Galactosemia
Path & Presentation |
Most common cause: deficiency of Galactose-1-phosphate-uridyltransferase
Less common and less severe: Galactokinase deficiency |
Pt. presents soon after starting breast-feeding with vomiting, lethargy, and failure to thrive.
Left untreated, will result in eye and liver damage. |
|
|
A formerly healthy infant presents at 8 months old who has been losing previously acquired motor function. Hepatosplenomegaly is noted on exam.
Liver biopsy reveals leukocytes with large, sphingomyeloid intracellular inclusions. |
Niemann-Pick Disease, a lysozomal storage disorder.
Sphingomyelinase deficiency results in accumulation of sphingomyelin in macrophages. Sphingomyelin deposition in CNS causes neurologic damage. |
Histo:
Foamy macrophages/histiocytes in liver and spleen |
|
|
Pt. is a 1.5 y/o presenting with a Gibbus, corneal clouding, coarse facial features (short, upturned nose, wide, upturned nostrils, macrocephaly), claw-hand deformity, and developmental slowness.
Urinalysis shows hi GAGs. Dx and Path: |
Hurler syndrome, AKA MPS I, a lysozomal storage disorder.
Mucopolysaccharidosis in which heparan sulfate and dermatan sulfate accumulate d/t deficiency of alpha-L-iduronidase |
|
|
|
Tay Sachs
Path: |
Hexosaminidase A deficiency results in lipid accumulation in neurons.
|
|
|
|
Pt. presents in early adolescence with inability to sweat, heat and cold intolerance, and angiokeratomas.
|
Fabry's disease, an X-linked recessive autosomal storage disorder.
Lysozomal storage disorder in which deficiency of alpha-galactosidase A results in buildup of ceramide trihexoside. Lysozomal byproducts build up especially in highly vascular tissues, resulting in vasculites. |
Late stages can present with renal, heart, and lung problems.
|
|
|
What is the process and enzyme responsible for the changes of color that we see in a healing bruise?
|
RBC's die in the bruised region, leaving a great deal of Hb at the site of injury that must be recycled.
Heme oxygenase converts this heme to biliverdin in the first step of this reaction. Biliverdin is a greenish pigment, and that's what we see in bruises. |
|
|
|
Why does the adrenal gland produce more epinephrine than norepinephrine?
|
In the final step of its synthesis, epinephrine is converted from norepinephrine by phenylethanolamine-N-methyltransferase (PNMT).
Transcription of PNMT is upregulated by cortisol, which the adrenal gland is bathed in. |
|
|
|
Mechanism of cataract formation in diabetes:
|
1. Chronic hyperglycemia causes too much glucose to be converted into sorbitol in lens cells.
2. Sorbitol builds up too quickly and cannot be converted into fructose by lens cells' modest levels of sorbitol dehydrogenase. 3. Sorbitol is large and cannot leave the cell unless broken down into fructose. Osmotic swelling results. 4. Swelling leads to degeneration and opacification -- cataracts! |
|
|
|
What is the difference between an endonuclease and an exonuclease?
|
Exonucleases remove nucleotides from the end of a DNA strand, i.e. when DNA Polymerase III corrects its mistakes during DNA synthesis.
Endonucleases remove nucleotides from the middle of a strand, as in NER. |
|
|
|
How is the proofreading activity of DNA Polymerase I unique among the other DNA Polymerase enzymes?
|
All three of the DNA-P enzymes have a 3'-5' exonuclease capability
DNA Polymerase III synthesizes DNA from 5' to 3' (of course), and proofreads backwards! (from 3' to 5') Only DNA Polymerase I has a 5' to 3' exonuclease capability. It uses this to replace RNA primers and damaged base pairs. |
|
|
|
Deficiency of what enzyme would result in NADH buildup in anaerobic glycolysis?
|
Pyruvate is converted to lactate by pyruvate dehydrogenase.
Without this reaction, we would not be able to produce lactate, which is necessary for replenishing the NAD+ reserve. |
|
|
|
What is a Nucleolus made of and what does it do?
|
Nucleoli are bundles of proteins and rDNA (DNA that encodes ribosomes), and rRNA.
Nucleoli the sites of ribosome synthesis, from soup to nuts. |
Ribosome synthesis is carried out by RNA Polymerase I.
|

