![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
Alcohols |
Contain the -OH functional group -OH responsible for the physical and chemical properties of the alcohols Suffix -ol to the name of the longest chain use a number to show position such as 2-methylbutane-2,3-diol |
|
|
Physical properties |
Compare to alkanes with the same length of carbon chain Alcohols are less volatile, higher melting points and greater water solubility than alkanes but differences are smaller as the length of the carbon chain increases Alkanes non-polar and very weak London forces Alcohols have a polar O-H bond because of their difference in electronegativity as the molecules are polar Alcohols have weak London forces but also much stronger hydrogen bonds between polar OH groups |
|
|
Volatility and boiling points |
Not very volatile compounds are not easily converted from a liquid to a gas and have a high boiling point Alcohols have a lower volatility and a higher boiling point than alkanes Because the alcohol has extra hydrogen bonding that requires more energy to overcome than the weak London forces As the chains get longer there is a smaller difference between alcohols and alkanes as the OH has a much smaller influence over a large molecule |
|
|
Solubility in water |
Alcohols can form hydrogen bonds with the water and alkanes can’t as they are non-polar Small alcohols are completely soluble in water as the OH group has a large influence over a small molecule but as the chain increases in length the influence of the OH decreases so solubility decreases |
|
|
Solubility in water picture |
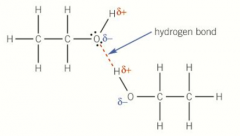
|
|
|
Classifying alcohols |
Primary, secondary or tertiary depending on how many hydrogen atoms and alkyl groups are attached to the carbon with the OH group Primary alcohols - OH attached to a C with 2 hydrogens and one alkyl group includes methanol which has 3 hydrogens attached Secondary alcohols - OH attached to a C with 1 hydrogen and 2 alkyl groups attached Tertiary alcohols - OH attached to a C with no hydrogen and 3 alkyl groups attached so OH opposite a branch |
|
|
Combustion of alcohols |
Alcohols burn completely in oxygen to produce carbon dioxide and water C2H5OH + 3O2 = 2CO2 + 3H2O Exothermic to produce large quantities of heat - as the carbon chain increases more heat is released per mole |
|
|
Oxidation of alcohols |
Primary and secondary alcohols can be oxidised by an oxidising agent usually potassium dichromate K2Cr2O7 acidified by dilute H2SO4 If the alcohol is oxidised it turns from orange to green Cr2O7 2- are orange to Cr3+ are green |
|
|
Oxidation of primary alcohols - aldehydes |
Primary alcohol oxidises with gentle heating produces an aldehyde Distil it to remove the aldehyde as soon as it has formed to prevent further oxidation Orange to green [O] indicates oxidising agent |
|
|
Oxidation of primary alcohols - aldehydes picture |
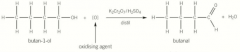
|
|
|
Oxidation of primary alcohols - carboxylic acids |
Primary alcohol heated strongly under reflux with excess acidified potassium dichromate then carboxylic acid formed Excess to ensure it has all been oxidised and reflux ensure any aldehyde formed undergoes oxidation again |
|
|
Oxidation of primary alcohols - carboxylic acids picture |
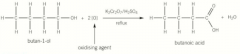
|
|
|
Oxidation of secondary alcohols |
Secondary alcohols oxidised to ketones and no possible further oxidation Heated under reflux to ensure the reaction has gone to completionOrange to green |
|
|
Oxidation of secondary alcohols picture |
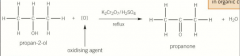
|
|
|
Oxidation of tertiary alcohols |
Tertiary alcohols do not undergo oxidation reactions Remains orange |
|
|
Dehydration of alcohols |
A water molecule is removed is dehydration and is an elimination reaction Heated under reflux with an acid catalyst such as concentrated H2SO4 or concentrated H3PO4 Produces an alkene |
|
|
Dehydration of alcohols picture |
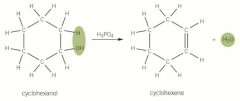
|
|
|
Substitution reactions of alcohols |
Alcohols react with hydrogen halides to form haloalkanes Heated under reflux with sulfuric acid and a sodium halide so the hydrogen halide is made in situ NaBr + H2SO4 = NaHSO4 + HBr Overall reaction = CH3CHOHCH3 + NaBr + H2SO4 = CH3CHBrCH3 + NaHSO4 + H2O |
|
|
Substitution reactions of alcohols picture |
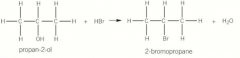
|

