![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
Acids in pharm are defined as? Or Drugs in medicine are said to be an acid if the uncharged (nonionized) compound is? |
the Proton donor (hydrogen ion(h+)) Ex: ASA, ibuprofen,PCN,Ketoralac, Barbiturates |
|
|
Bases in pharm are defined as? Or Drugs in medicine are said to be a base if the uncharged (nonionized) compound is? |
the Proton acceptor Ex: Ketamine,Etomidate, Local Anesthetic,Opiods |
|
|
Acid/Base reactions form what kind of products |
Conjugate acids and Conjugate Bases + - NH3+H2O<> NH4 + OH base acid conj. acid conj. base |
|
|
+ - Ka= [H ][Cl ]/[HCl]=acid dissociation constant |
The larger the Ka the stronger the acid |
|
|
"p" in front of something means what? |
"-log" (Inverse log) |
|
|
What does a larger Ka mean? What does a larger pKa mean? |
Stronger acid Stronger base pKa is drug specific and does not change |
|
|
What is an Ionization Fraction |
For HA <> H+ + A- the fraction (or %) of a drug that exists in the ionized state
Ionization Fraction= [A- ]/[HA]+[A-] If pH = pKa, then the ionization fraction is 50% |
|
|
Which is more Ionized (Stronger Acid) -4 Lactic acid Ka=1.3x10 -5 Acetic acid Ka=1.7x10 |
Lactic acid as the Ka is a larger number or stronger acid |
|
|
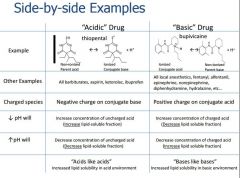
Which is more Ionized (Stronger Acid)
Thiopental pKa= 8.4 Methohexital pKa=8.0 |
Methohexital as the pKa is a smaller number or a stronger acid |
|
|
Which acid drug has a higher ionization fraction?
Ibuprofen pKa=4.9 Aspirin pKa=3.5 |
Aspirin as the pKa is a smaller number or a stronger acid which will have a higher percentage of drug in the ionized state. |
|
|
Which basic drug has a higher ionization fraction? Lidocaine pKa=7.8 Bupivicaine pKa=8.1 |
Bupivicaine, for basic drugs the stronger the acid (smaller pKa) the lower the ionization fraction. The base stays charged (or ionized) by not giving up the proton (or H+) |
|
|
Le Chatelier's Principle |
a chemical system at equillibrium will respond to stresses by readjusting itself to (partially) counteract the change. |
|
|
Le Chatelier's Principle -4 Lactic Acid <> Lactate- + H+ (Ka=1.3x10 ) What will happen if you add more Lactic Acid? |
The system will counteract by making more Lactate- and H+ to try to keep Ka constant. |
|
|
Do Ionized or nonionized drugs cross the Blood Brain Barrier? |
Nonionized as they need to be lipid soluble. |
|
|
How does sodium bicarbonate affect local anesthetics like Lidocaine? Local anesthetics block Na+ transmission of electrical impulses at the intracellular site of the channel. |
Adding a base binds protons decreasing the ionized amount of lidocaine available by Le Chatlier's principle which increases the nonionized lidocaine, allowing more to enter the cell across the phospholipid bilayer to affect Na transmission increasing the pain control |
|
|

|

