![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
Why is e.m.f the same when operating in alkaline or acidic conditions? |
Overall reaction is the same |
|
|
Define transition metal |
A metal that can form one or more stable ions with a partially filled d-subshell. |
|
|
Why is electron affinity exothermic? |
Net attraction between nucleus and added electron. |
|
|
Why does entropy increase in terms of movement of particles? |
Molecules VIBRATE more, so more disorder. |
|
|
Why do complex ions become more stable in reaction? |
Positive entropy change, so increase in number of particles. |
|
|
How do T-metal catalysts work? |
By changing oxidation states by gaining or losing electron in d orbital. |
|
|
Word for excessive amount eg blood fluid |
Plethora |
|
|
What agent is zinc? |
Reducing agent. It can reduce Cr3+ to Cr2+ |
|
|
Name 3 physical properties for Transition metals |
- High density - High melting and Boiling points - All very similar ionic radii |
|
|
Name 3 chemical properties for transition metals |
- Form complex ions (bonds to ligands) - Form coloured ions - Good catalysts |
|
|
What is a complex ion |
A metal ion surrounded by co-ordinately bonded ligands |
|
|
Spectrometry used to find concentrations of transition metal ions. How? |
- White light shone through filter - Filter only let's colour of light through - Colour of light passes through sample then to colorimeter. - Colorimeter calculates how much light absorbed by sample. - More light absorbed = higher concentration of ions |
|
|
Cr2O7(2-) in alkali, OH-, forms |
2CrO4(2-) + H+ This is reverse reaction, exists in equilibrium |
|
|
Dichromate, Cr2O7(2-), can be reduced by good reducing agent?
What's the equation |
- Zinc and dilute acid
Cr2O7(2-) + 3Zn + 14H+ --> 3Zn(2+) + 2Cr(3+) + 7H2O |
|
|
Zinc reduces Cr(3+) to... |
Cr(2+)
2Cr(3+) + Zn --> Zn(2+) + 2Cr(2+) Green/violet Blue
Requires inert atmosphere because Cr(2+) is very unstable it will oxidise back to Cr(3+) |
|
|
Can oxidise Cr(3+) to CrO4(2-) using H2O2 in alkaline |
2Cr(3+) + 10OH(-) + 3H2O2 --> 2CrO4(2-) + 8H2O |
|
|
Equation to oxidise Co(2+) to Co(3+)? Co(2+) is more stable |
2Co(2+) + H2O2 --> 2Co(3+) + 2OH- |
|
|
[Co(H2O)4(OH)2] - blue precipitate can also be written as |
Co(OH)2 |
|
|
Increasing surface area of heterogeneous catalyst |
Increases number of molecules that can react at the same time, so increases rate of reaction
Cos reaction happens on surface of the catalyst |
|
|
How can you make catalysts more effective |
Use support mediums. For example ceramic lattice coated in rhodium is used in catalytic converters. Minimises cost because only thin layer of rhodium catalyst used |
|
|
The 3 examples of heterogeneous catalysts are: |
- Iron (Fe) in Haber process, N2 + 3H2 --> 2NH3 - V2O5, Contact process making sulfuric acid, SO2 + (1/2)O2 --> SO3 - Cr2O3 making methanol from CO, 2H2 + CO --> CH3OH |
|
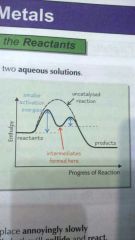
Explain how homogeneous catalysts work |
Homogeneous catalysts forms intermediate species. Catalyst reacts with reactants to form intermediate which then reacts with product to reform catalyst. Activation energy for this is lower than to form products from reactants. |
|
|
Homogeneous autocatalysis definition and example |
- Product of reaction acts as a catalyst so as reaction progresses, product increases so reaction speeds up 2MnO4(-) + 16H+ + 5C2O4(2-) --> 2Mn(2+) + 8H2O + 10CO2 |
|
|
Metal that's easier to oxidise, lose electrons, has a more |
Negative electrode potential
|
|
|
Half cell representation - more negative half cell (oxidised) starts on |

Left, with more positive reduced on the right |
|

Strongest reducing and strongest oxidising agent? |
Strongest oxidising = MnO4(-) - most positive left hand substance
Strongest reducing = Mg - most negative right hand substance |
|
|
Why would industrial companies carry out reactions higher than feasible temperature |
To speed reaction up |
|
|
Describe a standard hydrogen electrode potential |
- hydrogen gas - 1.00 mol dm-3 - 298k and 100kpa - platinum electrode |
|
|
Oxidation (negative cathode) and reduction (positive cathode) happens on which parts of cell convention |
Oxidation (negative cathode) - LEFT
reduction (positive cathode) - RIGHT MOST NEGATIVE E/V TO MOST POSITIVE E/V |
|
|
Metals and non metals are best for which agents |
Metals are better reducing agents because they like to lose electrons and get oxidised so more reactivate metals have more negative electrode potentials - reducing agent Non metals are better oxidising agents because they like to gain electrons so more reactive non metals have more positive electrode potentials, oxidising agents |
|
|
Mg reacts with steam to form? And what is the product and its colour |
MgO + H2 Brilliant white flame
MgO = white solid/ash |
|
|
Na2O forms what flame and what is Na2O |
Yellow flame White ash |
|
|
When a question contains DILUTE and AMPHOTERIC for when writing an equation you must include ... In the reactants |
Water - H2O |
|
|
[Fe(H2O)]2+ + NH3 forms |
NH4+ and Fe(H2O)4(OH)2 |
|
|
Mean bond enthalpies for what type of molecules are the same? |
Diatomic |
|
|
Mg(OH)2 is a weak alkaline solution, why? |
It's not very soluble in water so few OH- ions are produced |
|
|
SiO2 is macromolecular so is insoluble but is it acidic or basic |
Acidic |
|
|
covalent oxides will neutralise... |
Alkaline because covalent oxides are acidic |
|
|
Writing equation for oxidation of complex ion requires what in reactants |
Oxygen and water |
|
|
What to remember when calculating enthalpy formation etc |
Divide answer by 2 if 2 moles formed in equation |
|
|
Bond enthalpy DATA |
arrows go down then up |
|
|
When reacting with ammonia for t-metal equations, what must be used because NH3 is basic |
OH- ions to get rid of H+ acid so OH- must be used in equation to make alkali due to NH3 being basic |
|
|
Bond enthalpy is .... Of atomisation |
Double atomisation |
|
|
Equilibrium moving to the side of the electrons makes the E/V more |
Negative - in relative to hydrogen equilibrium |

