![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
485 Cards in this Set
- Front
- Back
|
characteristics of skeletal cartilage |
water lends resiliency contains no blood vessels surrounded by perichondrium hyaline elastic fibrocartilage |
|
|
perichondrium |
dense connective tissue girdle surrounds skeletal cartilage contains blood vessels for nutrient delivery resists outward expansion
|
|
|
3 types of skeletal cartilage |
hyaline cartilage elastic cartilage fibrocartilage
|
|
|
same basic components of all three skeletal cartilages |
contains chondrocytes (cells), encased in small cavities called lacunae, within an extracellular matrix (jellylike ground substance and fibers) |
|
|
hyaline cartilage |
support, flexibility and resilience collagen fibers only (most abundant) articular, costal, respiratory, nasal cartilage |
|
|
articular cartilage |
hyaline cartilage covers ends of most bones at movable joints |
|
|
costal cartilages
|
hyaline cartilage connect ribs to the sternum |
|
|
respiratory cartilages |
hyaline cartilage form skeleton of larynx (voicebox) reinforce other respiratory passageways |
|
|
nasal cartilages |
hyaline cartilage support the external nose |
|
|
elastic cartilage |
skeletal cartilage similar to hyaline, but contains elastic fibers external ear and epiglottis |
|
|
epiglottis |
flap that bends to cover the opening of the larynx each time we swallow |
|
|
fibrocartilage |
skeletal cartilage thick collagen fibers great tensile strength menisci of knee vertebral discs |
|
|
two ways cartilage can grow |
appositional growth interstitial growth |
|
|
appositional growth |
growth of cartilage cartilage forming cells in perichondrium secrete matrix against external face of existing cartilage |
|
|
interstitial growth |
cartilage growth chondrocytes divide and secrete new matrix, expanding cartilage from within |
|
|
calcification of cartilage |
occurs during normal bone growth youth and old age cartilage hardens due to deposit of calcium salts (calcified cartilage is NOT bone) |
|
|
how many named bones in skeleton |
206 |
|
|
2 groups of bones |
axial skeleton appendicular skeleton |
|
|
axial skeleton |
long axis of body bones of skull, vertebral column and rib cage |
|
|
appendicular skeleton |
bones of upper and lower limbs girdles (shoulder and hip bones) attaching limbs to axial skeleton |
|
|
4 bone shapes |
long short flat irregular |
|
|
long bones |
longer than they are wide all limb bones except patella, wrist and ankle |
|
|
short bones |
cube shaped bones (wrist and ankle) sesamoid bones (within tendons) vary in size/number in different individuals |
|
|
sesamoid bones |
special type short bone forms in tendon patella |
|
|
flat bones |
thin, flat, slightly curved sternum, scapulae, ribs, most skull bones |
|
|
irregular bones |
complicated shapes vertebrae, coxal bones (hip bones) |
|
|
7 functions of bones |
1. support 2. protection 3. movement 4. mineral and growth factor storage 5. blood cell formation 6. triglyceride (fat) storage 7. hormone production |
|
|
functions of bones: support |
framework supports body cradles soft organs |
|
|
functions of bones: protection |
skull: protects brain vertebrae bones: protect spinal cord rib cage: protects vital organs |
|
|
functions of bones: movement |
skeletal muscles use bones as levers for muscle action (to move body and its parts) |
|
|
functions of bones: mineral and growth factor storage |
bone reservoir for minerals calcium and phosphorus bone matrix stores growth factors |
|
|
functions of bones: blood cell formation (hematopoiesis) |
occurs in red marrow cavities of bones |
|
|
hematopoiesis |
blood cell formation |
|
|
functions of bones: triglyceride (fat) storage |
storage in bone cavities energy source |
|
|
functions of bones: hormone production |
bones produce osteocalcin |
|
|
osteocalcin |
hormone produced by bones helps regulate bone formation protects against obesity, glucose intolerance and diabetes mellitus |
|
|
why are bones organs |
they contains different types of tissues |
|
|
different types of tissues in bones (6) |
bone (osseous tissue) nervous tissue (in nerves) cartilage tissue fibrous connective tissue (lining cavities) muscle and epithelial tissues (in blood vessels) |
|
|
3 levels of bone structure |
gross anatomy microscopic chemical |
|
|
gross anatomy |
bone textures structure of short, irregular and flat bones structure of typical long bone location of hematopoietic tissues in bones bone markings |
|
|
2 bone textures |
compact spongy |
|
|
compact bone |
dense outer layer smooth and solid |
|
|
spongy bone |
also called trabecular bone internal to compact bone honeycomb of flat pieces of bone deep to compact ^-- called trabeculae |
|
|
trabeculae |
spongy bone a honeycomb of small-needle like or flat pieces |
|
|
structure of short, irregular and flat bones (5) |
1. thin plates of spongy bone covered by compact bone 2. plates sandwiched between connective tissue membranes: periosteum (outer layer) and endosteum 3. no shaft or epiphyses 4. contain bone marrow throughout spongy bone but no marrow cavity 5. hyaline cartilage covers articular surfaces |
|
|
structure of typical long bone |
most long bones have same general structure a shaft, bone ends and membranes diaphysis and epiphyses |
|
|
diaphysis |
(structure of typical long bone) tubular shaft forms long axis of bone compact bone surrounding medullary cavity |
|
|
medullary cavity |
also called marrow cavity long bone surrounded by thick collar of compact bone in adults, it contains fat (yellow marrow) and is called yellow marrow cavity |
|
|
epiphyses |
(structure of typical long bone) bone ends external compact bone forms epiphyses exterior internal epiphyses contains spongy bone articular cartilage covers articular surfaces between epiphyses and diaphysis is epiphyseal line |
|
|
epiphyseal line |
between diaphysis and epiphysis of bone remnant of childhood bone growth at epiphyseal plate |
|
|
epiphyseal plate |
disc of hyaline cartilage that grows during childhood to lengthen the bone |
|
|
2 membranes of long bone |
periosteum endosteum |
|
|
periosteum (structure of typical long bone) |
white, double-layered membrane covers external surfaces (except joint surfaces) outer fibrous layer is dense irregular connective tissue osteogenic layer abuts bone many nerve fibers and blood vessels anchoring points for tendons and ligamets |
|
|
sharpey's fibers |
periosteum membrane collagen fibers that extend from fibrous layer into the bone matrix secure periosteum to underlying bone |
|
|
osteogenic layer of periosteum |
abuts bone surface contains primitive stem cells (osteogenic cells) |
|
|
endosteum (structure of typical long bone) |
delicate connective tissue membrane covers internal bone surface covers trabeculae of spongy bone lines canals that pass through compact bone contains osteogenic cells that can differentiate into other bone cells |
|
|
hematopoietic tissue in bones (structure of typical long bone) |
hematopoietic tissue= red marrow found in trabecular cavities of spongy bone found in diploe of flat bones found in medullary cavities and spongy bone of newborns adult long bones have little red marrow (heads of femur and humerus only) red marrow in diploe and irregular bones is most active yellow marrow can convert to red if necessary |
|
|
blood cell production (hematopoiesis) in adult long bones routinely occurs only in |
the heads of the femur and humerus |
|
|
bone markings (structure of typical long bone) |
sites of muscle, ligament and tendon attachment on external surfaces joint surfaces conduits for blood vessels and nerves projections, depressions and openings |
|
|
bone markings: projections |
most indicate stresses created by muscle pull of joint modifications |
|
|
bone markings: depressions and openings |
allow nerves and blood vessels to pass |
|
|
microscopic anatomy of bone: 5 major cell types of bone tissue |
each specialized form of same basic cell type osteogenic cells osteoblasts osteocytes bone lining cells osteoclasts |
|
|
osteogenic cells |
also called osteoprogenitor cells cells of bone tissue mitotically active stem cells in periosteum and endosteum when stimulated, differentiate into osteoblasts or bone lining cells (though some persist as osteogenic cells) |
|
|
osteoblasts |
cells of bone tissue bone-forming cells secrete unmineralized bone matrix (osteoid) actively mitotic |
|
|
osteoid |
unmineralized bone matrix secreted by osteoblasts include collagen (90% of bone protein) and calcium binding proteins |
|
|
osteocytes |
cells of bone tissue mature bone cells in lacunae monitor and maintain bone matrix act as stress or strain sensors |
|
|
how do osteocytes act as stress or strain sensors |
respond to and communicate mechanical stimuli to osteoblasts and osteoclasts so bone remodeling can occur |
|
|
bone lining cells |
cells of bone tissue flat cells on bone surfaces believed to help maintain matrix periosteal cells and endosteal cells |
|
|
periosteal cells |
bone lining cells on external bone surface |
|
|
endosteal cells |
bone lining cells lining internal bone surfaces |
|
|
osteoclasts |
cells of bone tissue bone-destroying cells derived from hematopoietic stem cells that become macrophages giant multinucleate cells for born resorption when active, rest in resorption bay and have ruffled border |
|
|
ruffled border of osteoclasts |
when active increases surface area for enzyme degradation of bone and seals off area from surrounding matrix |
|
|
microscopic anatomy of bone: compact bone |
(lamellar bone) contains passageways that serve as conduits for nerves and blood vessels osteon or haversian system canals and canaliculi interstitial and circumferential lamellae |
|
|
osteon or haversian system |
structural unit of compact bone each osteon is a elongated cylinder parallel to long axis of bone hollow tubes of bone matrix called lamellae collagen fibers in adjacent rings run different directions which resists twisting |
|
|
microscopic anatomy of bone: compact bone- canals and canaliculi |
central (haversian) canal perforating (volkmann's) canal lacunae canaliculi |
|
|
central (haversian) canal |
compact bone runs through core of osteon contains blood vessels and nerve fibers
|
|
|
perforating (volkmann's) canals |
compact bone canals lined with endosteum at right angles to central canal connect blood vessels and nerves of periosteum, medullary cavity and central canal |
|
|
compact bone: lacunae |
small cavities that contain osteocytes |
|
|
canaliculi |
compact bone hairlike canals that connect lacunae to each other and central canal |
|
|
canaliculi formation in compact bone |
osteoblasts secreting bone matrix maintain contact with eachother and osteocytes via cell projections with gap junctions canaliculi forms when matrix hardens and cells are trapped allows communication permits nutrients and wastes to be relayed from one osteocyte to another throughout osteon |
|
|
interstitial lamellae |
compact bone incomplete lamellae not part of complete osteon fill gaps between forming osteons remnants of osteons cut by bone remodeling |
|
|
circumferential lamellae |
compact bone just deep to periosteum superficial to endosteum extend around entire surface of diaphysis resist twisting of long bone |
|
|
microscopic anatomy of bone: spongy bone |
appears poorly organized trabeculae: 1. align along lines of stress to help resist it 2. no osteons 3. contain irregularly arranged lamellae and osteocytes interconnected by canaliculi 4. capillaries in endosteum supply nutrients |
|
|
chemical composition of bone |
both organic and inorganic substances organic components: bone cells and osteoid inorganic components: mineral salts |
|
|
organic components of bones: cells (5) and osteoid |
1. osteogenic cells 2. osteoblasts 3. osteocytes 4. bone lining cells 5. osteoclasts osteoid: 1/3 of organic bone matrix secreted by osteoblasts made of ground substance collagen fibers contributes to structure provides tensile strength and flexibility |
|
|
sacrificial bonds |
contributes to resilience of bone in or between collagen molecules stretch and break easily on impact to dissipate energy and prevent fracture (bonds reform) |
|
|
hydroxyapatites |
inorganic mineral salts in bone 65% of bone by mass mainly tiny calcium phosphate crystals in and around collagen fibers responsible for hardness and resistance to compression |
|
|
hydroxyapatites (mineral salts) are responsible for |
hardness of bone and resistance to compression |
|
|
characteristics of bone (5) |
1. half as strong as steel in resisting compression 2. as strong as steel in resisting tension 3. last long after death because of mineral composition 4. reveal information about ancient people 5. can display growth arrest lines |
|
|
growth arrest lines |
horizontal lines on bones proof of illness: when bones stop growing so nutrients can help fight disease |
|
|
ossification |
(osteogenesis) process of bone tissue formation formation of bony skeleton, postnatal bone growth, bone remodeling and repair |
|
|
formation of bony skeleton begins in |
2nd month of development |
|
|
postnatal bone growth occurs until |
early adulthood |
|
|
bone remodeling and repair occurs |
your whole life |
|
|
two types of ossification |
endochondral ossification intramembranous ossification |
|
|
general characteristics of endochondral ossification (3) |
1. bone forms by replacing hyaline cartilage 2. bones called cartilage (endochondral) bones 3. forms most of skeleton |
|
|
general characteristics of intramembranous ossification (3) |
1. bone develops from fibrous membrane 2. bones called membrane bones 3. forms flat bones (clavicles and cranial bones) |
|
|
what type of ossification forms most all bones inferior to base of skull (except clavicles) |
endochondral ossification |
|
|
endochondral ossification requires breakdown of what prior to ossification |
hyaline cartilage |
|
|
endochondral ossification begins at |
primary ossification center in center of shaft blood vessel infiltration of perichondrium converts it to periosteum underlying cells change to osteoblasts |
|
|
first stage of endochondral ossification |
bone collar forms around diaphysis of hyaline cartilage model |
|
|
2nd stage of endochondral ossification |
central cartilage in diaphysis calcifies and then develops cavities |
|
|
3rd stage of endochondral ossification |
periosteal bud invades internal cavities and spongy bone forms |
|
|
4th stage of endochondral ossification |
diaphysis elongates and a medullary cavity forms |
|
|
5th stage of endochondral ossification |
the epiphyses ossify |
|
|
periosteal bud |
collection of elements that invades internal cavities during 3rd stage of endochondral ossification contains a nutrient artery and vein, nerve fibers, red marrow elements, osteogenic cells and osteoclasts |
|
|
intramembranous ossification forms what bones |
frontal, parietal, occipital, temporal bones, and clavicles |
|
|
intramembranous ossification begins within |
fibrous connective tissue membranes formed by mesenchymal cells |
|
|
1st stage of intramembranous ossification |
ossification centers appear in fibrous connective tissue membrane |
|
|
2nd stage of intramembranous ossification |
osteoid is secreted within fibrous membrane and calcifies |
|
|
3rd stage of intramembranous ossification |
woven bone and periosteum form |
|
|
4th stage of intramembranous ossification |
lamellar bone replaces woven bone, just deep to periosteum red marrow appears |
|
|
2 growth methods of postnatal bone growth |
interstitial (longitudinal) appositional |
|
|
increase in length of long bones |
interstitial (longitudinal) growth |
|
|
increase in bone thickness |
appositional growth |
|
|
type of growth that requires presence of epiphyseal cartilage |
interstitial growth (growth in length of long bones) |
|
|
what remains at a constant thickness during interstitial growth? why? |
epiphyseal plate rate of cartilage growth on one side balanced by bone replacement on other |
|
|
5 zones within cartilage- interstitial growth |
resting (quiescent) zone proliferation (growth) zone hypertrophic zone calcification zone ossification (osteogenic) zone |
|
|
resting (quiescent) zone |
(interstitial growth) cartilage on epiphyseal side of epiphyseal plate relatively inactive |
|
|
proliferation (growth) zone |
(interstitial growth) cartilage on diaphysis side of epiphyseal plate rapidly divide pushing epiphysis away from diaphysis----> lengthening |
|
|
hypertrophic zone |
(interstitial growth) older chondrocytes closer to diaphysis and their lacunae enlarge and erode----> interconnecting spaces |
|
|
calcification zone |
(interstitial growth) surrounding cartilage matrix calcifies, chondrocytes die and deteriorate |
|
|
ossification zone |
(interstitial growth) chondrocyte deterioration leaves long spicules of calcified cartilage at epiphysis-diaphysis junction spicules eroded by osteoclasts covered with new bone by osteoblasts ultimately replaced with spongy bone |
|
|
near end of adolescence, what divides less often |
chondroblasts |
|
|
near end of adolescence epiphyseal plate thins and is replaced by |
bone |
|
|
what happens during epiphyseal plate closure (interstitial growth) |
bone lengthening ceases which requires presence of cartilage bone of epiphysis and diaphysis fuses |
|
|
when does epiphyseal plate closure occur in females |
about 18 years |
|
|
when does epiphyseal plate closure occur in males |
about 21 years |
|
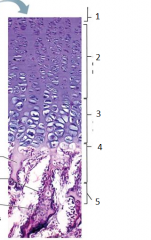
what is this |
epiphyseal plate |
|
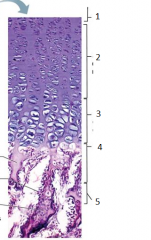
1 |
resting (quiescent zone) |
|
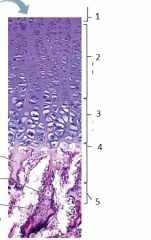
2 |
proliferation (growth) zone cartilage cells undergo mitosis |
|
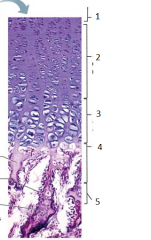
3 |
hypertrophic zone older cartilage cells enlarge |
|
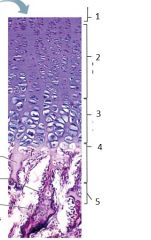
4 |
calcification zone matrix calcifies cartilage cells die matrix begins deteriorating blood vessels invade cavity |
|
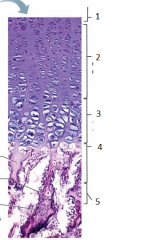
5 |
ossification zone new bone forms |
|
|
appositional growth: main points |
allows lengthening bone to widen occurs throughout life |
|
|
during appositional growth, osteoblasts do what |
osteoblasts beneath periosteum secrete bone matrix on external bone |
|
|
during appositional growth what do osteoclasts do |
osteoclasts remove bone on endosteal surface |
|
|
hormonal regulation of bone growth: growth hormone |
most important in stimulating epiphyseal plate activity in infancy and childhood |
|
|
hormonal regulation of bone growth: thyroid hormone |
modulates activity of growth hormone ensures proper proportions |
|
|
hormonal regulation of bone growth: testosterone and estrogens (at puberty) |
promote adolescent growth spurts end growth by inducing epiphyseal plate closure |
|
|
excesses or deficits of any hormone for bone growth cause |
abnormal skeletal growth |
|
|
how much bone mass is recycled each week |
5-7% |
|
|
spongy bone is replaced every |
3-4 years |
|
|
compact bone is replaced every |
10 years |
|
|
why does older bone become more brittle |
calcium salts crystallize fractures more easily |
|
|
bone remodeling |
consists of bone deposit and bone resorption occurs at surfaces of both periosteum and endpsteum remodeling units |
|
|
remodeling units |
coordinate bone remodeling packets of adjacents osteoblasts and osteoclasts
|
|
|
bone deposit |
evidence of new matrix deposit by osteoblasts osteoid seam calcification front trigger of calcification not confirmed |
|
|
osteoid seam |
unmineralized band of bone matrix marks areas of new matrix deposits by osteoblasts |
|
|
calcification front |
abrupt transition zone between osteoid seam and older mineralized bone |
|
|
bone resorption is accomplished by what |
osteoclasts |
|
|
osteoclasts and bone resorption |
dig depressions or grooves as break down matrix secrete lysosomal enzymes that digest matrix and protons acidity converts calcium salts to soluble forms once resorption complete osteoclasts undergo apoptosis |
|
|
bone resorption: osteoclasts and phagocytosis |
osteoclasts phagocytize demineralized matrix and dead osteocytes transcytosis allow release into interstitial fluid and then into blood |
|
|
osteoclast activation during bone resorption involves what |
PTH and T cell-secreted proteins |
|
|
bone remodeling occurs continuously but regulated by |
genetic factors and two control loops |
|
|
2 control loops that regulate bone remodeling |
1. negative feedback hormonal loop that maintains calcium homeostasis in blood 2. responses to mechanical and gravitational forces acting on skeleton |
|
|
5 important functions of calcium |
1. nerve impulse transmission 2. muscle contraction 3. blood coagulation (clotting of blood) 4. secretion by glands and nerve cells 5. cell division |
|
|
how much calcium in body |
1200-1400 grams 99% as bone minerals |
|
|
2 primary hormonal controls of calcium in blood |
parathyroid hormone (PTH) calcitonin |
|
|
hormonal control of calcium in blood: parathyroid hormone (PTH) |
produced by parathyroid glands removes calcium from bone regardless of bone integrity |
|
|
hormonal control of calcium in blood: calcitonin |
produced by parafollicular cells of thyroid gland in high doses lowers blood calcium levels temporarily |
|
|
calcium homeostasis |
even minute changes in blood calcium dangerous severe neuromuscular problems hypercalcemia |
|
|
2 severe neuromuscular problems from change in blood calcium |
hyperexcitability nonresponsiveness |
|
|
hyperexcitability |
when blood calcium levels are too low |
|
|
nonresponsiveness |
when blood calcium levels are too high |
|
|
hypercalcemia |
sustained high blood calcium levels deposits of calcium salts in blood vessels, kidneys can interfere with function |
|
|
2 hormones that affect bone density |
leptin serotonin |
|
|
leptin |
hormone released by adipose tissue role in bone density regulating inhibits osteoblasts in animals |
|
|
serotonin |
neurotransmitter regulating mood and sleep most made in gut secreted into blood after eating interferes with osteoblast activity serotonin reuptake inhibitors (prozac) cause lower bone density |
|
|
bone homeostasis: response to mechanical stress |
bones reflect stresses they encounter bones stressed when weight bears on them or muscles pull on them |
|
|
wolff's law |
bones grow or remodel in response to demands placed on it |
|
|
5 examples of wolff's law |
1. handedness (left or right handed) results in thicker and stronger bone of upper limb 2. curved bones thickest where most likely to buckle 3. trabeculae form trusses along lines of stress 4. large, bony projections occur where heavy, active muscles attach 5. bones of fetus and bedridden featureless |
|
|
how does mechanical stress cause bone remodeling |
electrical signals produced by deforming bone cause remodeling fluid flows within canaliculi appear to provide remodeling stimulus |
|
|
results of hormonal and mechanical influences (2) |
1. hormonal controls determine whether and when remodeling occurs to changing blood calcium levels 2. mechanical stress determines where remodeling occurs |
|
|
fractures |
breaks in bones youth: most result from trauma old age: most result of weakness from bone thinning |
|
|
3 classifications of fractures |
1. position of bone ends after fracture 2. completeness of break 3. whether skin is penetrated |
|
|
classification of fractures: position of bone ends after fracture |
nondisplaced: ends retain normal position displaced: ends out of normal alignment |
|
|
classification of fractures: completeness of break |
complete: broken all the way through incomplete: not broken all the way through |
|
|
classification of fractures: whether skin is penetrated |
open (compound): skin is penetrated closed (simple): skin in not penetrated |
|
|
in addition to the 3 classifications of fractures, bone fractures also described by what 3 things |
1. location of fracture 2. external appearance 3. nature of break |
|
|
treatment of a fracture involves |
reduction and immobilization |
|
|
reduction |
involved in treatment of fracture realignment of broken bone ends closed and open reductions |
|
|
closed (external) reduction |
physician manipulates to correct position |
|
|
open (internal) reduction |
bone ends secured surgically with pins or wires |
|
|
immobilization (treatment of fractures) |
after broken bone reduced, immobilized by cast or traction to allow healing depends on break severity, bone broken, and age of patient |
|
|
4 major stages of bone repair |
1. a hematoma forms 2. fibrocartilaginous callus forms 3. bony callus forms 4. bone remodeling occurs |
|
|
1st stage of bone repair |
(hematoma forms) torn blood vessels hemorrhage clot (hematoma) forms site swollen, painful, and inflamed |
|
|
hematoma |
mass of clotted blood |
|
|
2nd stage of bone repair |
(fibrocartilaginous callus forms) capillaries grow into hematoma phagocytic cells clear debris fibroblasts secrete collagen fibers to span break and connect broken ends fibroblasts, cartilage, and osteogenic cells begin reconstruction of bone mass of repair tissue called fibrocartilaginous callus |
|
|
during the 2nd stage of bone repair, what 3 things begin the reconstruction of the bone. how? |
fibroblasts, cartilage, and osteogenic cells they create cartilage matrix of repair tissue osteoblasts form spongy bone within matrix |
|
|
3rd stage of bone repair |
(bony callus forms) within one week new trabeculae appear in fibrocartilaginous callus callus converted to bony (hard) callus of spongy bone about 2 months later firm union forms |
|
|
4th stage of bone repair |
(bone remodeling occurs) begins during bony callus formation continues for several months excess material on diaphysis exterior and within medullary cavity removed compact bone laid down to reconstruct shaft walls final structure resembles original because responses to same mechanical stressors |
|
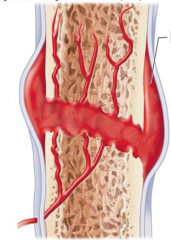
what stage is this |
stage 1 hematoma forms |
|
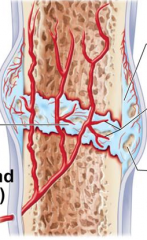
what stage is this |
stage 2 fibrocartilaginous callus forms |
|
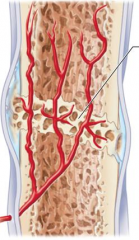
what stage is this |
stage 3 bony callus forms |
|
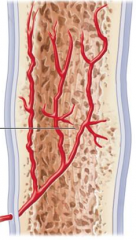
what stage is this |
stage 4 bone remodeling occurs
|
|
|
the diseases of the human skeleton are caused by |
imbalances between bone deposit and bone resorption |
|
|
3 disease of the human skeleton |
osteomalacia rickets osteoporosis |
|
|
osteomalacia |
(bone disease) bones poorly mineralized calcium salts not adequate soft, weak bones pain upon bearing weight |
|
|
rickets |
(bone disease) osteomalacia of children bowed legs and other bone deformities bones ends enlarged and abnormally long the cause: vitamin D deficiency or insufficient dietary calcium |
|
|
osteoporosis |
group of diseases bone resorption outpaces bone deposit spongy bone of spine and neck of femur most susceptible (why vertebral and hip fractures common) |
|
|
risk factors for osteoporosis for women |
most often aged, postmenopausal women 30% 60-70 years of age 70% by age 80 30% caucasian women will fracture bone because of itq |
|
|
risk factors for osteoporosis for men |
men lesser degree than women |
|
|
risk factors for osteoporosis: sex hormones |
sex hormones maintain normal bone health and density as secretion wanes with age, osteoporosis can develop |
|
|
7 additional risk factors for osteoporosis |
1. petite body form 2. insufficient exercise to stress bones 3. diet poor in calcium and protein 4. smoking 5. hormone related conditions 6. immobility 7. makes with prostate cancer taking androgen-suppressing drugs |
|
|
3 hormone related conditions that are risk factors for osteoporosis |
1. hyperthyroidism 2. low blood levels of thyroid-stimulating hormone 3. diabetes mellitus |
|
|
4 traditional treatments for osteoporosis |
1. calcium 2. vitamin D supplements 3. weight-bearing exercise 4. hormone replacement therapy |
|
|
hormone replacement therapy for osteoporosis |
slows bone loss but does not reverse it controversial due to increased risk of heart attack, stroke and breast cancer some take estrogenic compounds in soy as substitute |
|
|
new drugs for osteoporosis treatment (4) |
1. bisphosphonates 2. selective estrogen receptor modulators 3. statins 4. denosumab |
|
|
new drugs for osteoporosis treatment: bisphosphonates |
decrease osteoclast activity and number partially reverse in spine |
|
|
new drugs for osteoporosis treatment: selective estrogen receptor modulators |
mimic estrogen without targeting breast and uterus |
|
|
new drugs for osteoporosis treatment: statins |
though for lowering cholesterol also increase bone mineral density |
|
|
new drugs for osteoporosis treatment: denosumab |
monoclonal antibody reduces fractures in men with prostate cancer improves bone density in elderly |
|
|
3 major ways to prevent osteoporosis |
1. plenty of calcium in diet in early adulthood 2. reduce carbonated beverage and alcohol consumption 3. plenty of weight-bearing exercise |
|
|
paget's disease |
excessive and haphazard bone deposit and resorption bone made fast and poorly (pagetic bone) rarely occurs before age 40 cause is unknown treatment includes calcitonin and biphosphonates |
|
|
pagetic bone |
bone made fast and poorly very high ratio of spongy to compact bone and reduced mineralization usually in spine, pelvis, femur and skull |
|
|
primary function of lever arm system |
increase force (work) applied to an object by altering the "mechanical avdantage" |
|
|
secondary function of lever arm system |
increase acceleration (force) applied to an object |
|
|
primary function of lever arm system equation |
force (in) X distance (in) = force (out) X distance (out) |
|
|
secondary function of lever arm system equation |
force applied = mass (of object) X angle speed X radius |
|
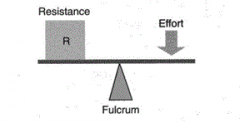
what is this |
first class lever arm system |
|
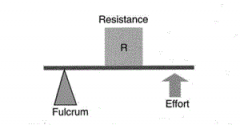
what is this |
second class lever arm system |
|
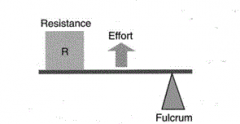
what is this |
third class lever arm system |
|
|
4 parts of skeletal system |
bones (skeleton) joints cartilage ligaments |
|
|
2 divisions of skeletal system |
axial skeleton appendicular skeleton |
|
|
paranasal sinuses |
hallow portions of bones surrounding nasal cavity frontal ethmoid sphenoid maxillary |
|
|
fetal skull large compared to |
the infants total body length |
|
|
ratio of fetal cranium to the facial skull compared to adults |
7:1 3:1 |
|
|
fontanelles |
fibrous membranes in fetal skull allow for compression during birth allow brain to grow convert to bone within 2 years |
|
|
characteristics of fetal skull |
pliable small face compared to cranium large brain fully developed inner ear eye sight |
|
|
vertebrae are separated by |
intervertebral discs |
|
|
each vertebrae is given name according to |
its location |
|
|
function of bony thorax |
forms a cage to protect major organs |
|
|
3 parts of bony thorax |
sternum ribs thoracic vertebrae |
|
|
pectoral (shoulder) girdle |
composed of 2 bones: clavicle and scapula allow the upper limb to have exceptionally free movement |
|
|
scapula and clavicle tightly anchor |
the brachial appendage to the axial skeleton |
|
|
carpals |
wrist |
|
|
metacarpals |
palm |
|
|
phalanges |
fingers |
|
|
bones of pelvic girdle |
hip bones (ilium, ischium, pubic bone) total weight of upper body rests in pelvis protects several organs |
|
|
3 fused hip bones |
ilium ischium pubic bone |
|
|
bones of the pelvic girdle protect what organs |
reproductive organs urinary bladder part of large intestine |
|
|
in a male pelvis, the pubic arch is |
less than 90 degrees |
|
|
in female pelvis, pubic arch is |
more than 90 degrees |
|
|
bones of the foot are arranged to form |
three strong arches two longitudinal one transverse |
|
|
in embryo, limb buds develop around |
week 4 |
|
|
in embryo, cartilage forms in the pectoral limb bud after how many weeks |
5 |
|
|
in an embryo, both appendages are recognizable as cartilaginous models of the adult bone after how many weeks |
10 |
|
|
muscle tissue transforms chemical energy (ATP) to |
directed mechanical energy (exerts force) |
|
|
three types of muscle tissue |
skeletal cardiac smooth |
|
|
3 prefixes for muscle |
myo mys sarco |
|
|
6 characteristics of skeletal muscle |
1. organs attached to bones and skin 2. elongated cells called muscle fibers 3. striated (striped) 4. voluntary (conscious control) 5. contract rapidly; tire easily; powerful 6. require nervous system stimulation |
|
|
4 characteristics of cardiac muscle |
1. only in heart; bulk of heart walls 2. striated 3. can contract without nervous system stimulation 4. involuntary |
|
|
4 characteristics of smooth muscle |
1. in walls of hallow organs (stomach, urinary bladder, airways) 2. not striated 3. can contract without nervous system stimulation 4. involuntary |
|
|
4 special characteristics of muscle tissue |
excitability contractility extensibility elasticity |
|
|
excitability |
(responsiveness) ability to receive and respond to stimuli |
|
|
contractility |
ability to shorten forcibly when stimulated |
|
|
extensibility |
ability to be stretched |
|
|
elasticity |
ability to recoil to resting length |
|
|
4 important functions of muscles |
1. movement of bones or fluids (blood) 2. maintaining posture and body position 3. stabilizing joints 4. heat generation (especially skeletal muscle) |
|
|
additional functions of muscles |
protects organs forms valves controls pupil size causes "goosebumps" |
|
|
each skeletal muscle is served by |
one artery, one nerve, and one or more veins |
|
|
every skeletal muscle fiber is supplied by |
nerve ending that controls its activity |
|
|
connective tissue sheaths of skeletal muscle |
support cells; reinforce whole muscle external to internal: epimysium perimysium endomysium |
|
|
epimysium |
dense irregular connective tissue surrounding entire muscle may blend with fascia |
|
|
perimysium |
fibrous connective tissue surrounding fascicles |
|
|
fascicles |
groups of muscle fibers |
|
|
endomysium |
fine areolar connective tissue surrounding each muscle fiber |
|
|
skeletal muscles attach at two places |
insertion origin |
|
|
insertion |
movable bone where skeletal muscle attaches |
|
|
origin |
immovable (less movable) bone where skeletal muscle attaches |
|
|
direct attachment of skeletal muscle |
epimysium fused to periosteum of bone or perichondrium of cartilage |
|
|
indirect attachment of skeletal muscles |
connective tissue wrappings extend beyond muscle as ropelike tendon or sheetlike aponeurosis |
|
|
sarcolemma |
plasma membrane of skeletal muscle fiber |
|
|
sarcoplasm |
cytoplasm of skeletal muscle fiber glycosomes for glycogen storage myoglobin for oxygen storage |
|
|
modified structures of skeletal muscle fibers |
myofibrils sarcoplasmic reticulum T tubules |
|
|
myofibrils |
densely packed, rodlike elements 80% of cell volume contain sacromeres exhibit striations |
|
|
sacromeres |
smallest contractile unit (functional unit) of muscle fiber align along myofibril like boxcars of train contains A band with half I band at each end |
|
|
striations of myofibrils |
perfectly aligned repeating series of dark A bands and light I bands |
|
|
H zone of myofibril striations |
lighter region in midsection of dark A band where filaments do not overlap |
|
|
M line of myofibril striations |
line of protein myomesin bisects H zone |
|
|
Z disc (line) of myofibril striations |
coin-shaped sheet of proteins on midline of light I band that anchors thin filaments and connects myofibrils to one another |
|
|
thick filaments of myofibril striations |
run entire length of an A band |
|
|
thin filaments of myofibril striations |
run length of I band and partway into A band |
|
|
sacromere of myofibril striations |
region between 2 successive Z discs |
|
|
sacromeres are composed of |
thick and thin myofilaments made of contractile proteins |
|
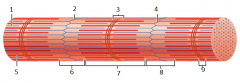
what is this |
myofibril |
|
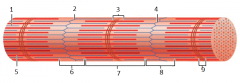
1 |
thin (actin) filament |
|
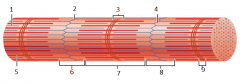
2 |
z-disc |
|
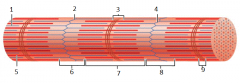
3 |
H zone |
|
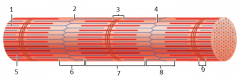
4 |
z disc |
|
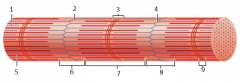
5 |
thick (myosin) filament |
|
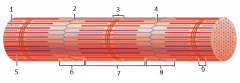
6 |
I band |
|
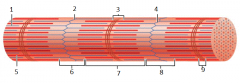
7 |
A band |
|
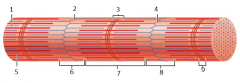
8 |
I band |
|
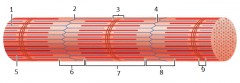
9 |
M line |
|
|
actin myofilaments |
thin filaments extend across I band and partway in A band anchored to Z discs |
|
|
myosin myofilaments |
thick filaments extend length of A band connected at M line |
|
|
structure of thick filament |
composed of protein myosin each composed of 2 heavy and 4 light polypeptide chains |
|
|
myosin tails |
contain 2 interwoven, heavy polypeptide chains |
|
|
myosin heads |
contain 2 smaller, light polypeptide chains that act as cross bridges during contraction |
|
|
cross bridges |
myosin heads binding sites for actin of thin filaments binding sites for ATP ATPase enzymes |
|
|
structure of thin filament |
twisted double strand of fibrous protein F actin F actin consists of G (globular) actin subunits G actin bears active sites for myosin head attachment during contraction tropomyosin and troponin |
|
|
tropomyosin and troponin |
regulatory proteins bound to actin |
|
|
structure of myofibril: elastic filament |
composed of protein titin holds thick filaments in place helps recoil after stretch resists excessive stretching |
|
|
structure of myofibril: dystrophin |
links thin filaments to proteins of sarcolemma |
|
|
structure of myofibril: nebulin, myomesin, C proteins |
bind filaments or sacromeres together maintain alignment |
|
|
sarcoplasmic reticulum (SR) |
network of smooth endoplasmic reticulum surrounding each myofibril most run longitudinally |
|
|
what forms perpendicular cross channels in sarcoplasmic reticulum |
pairs of terminal cisterns |
|
|
function of sarcoplasmic reticulum |
regulation of intracellular calcium levels stores and releases calcium |
|
|
T tubules |
continuations of sarcolemma lumen continuous with extracellular space increase muscle fiber's surface area |
|
|
T tubules penetrate cell's interior at each |
A band-I band junction |
|
|
T tubules associate with paired terminal cisterns to form |
triads that encircle each sacromere |
|
|
SR foot proteins |
gated channels that regulate calcium release from SR cisterns |
|
|
in relaxed state, thin and thick filaments overlap only at |
ends of A band |
|
|
contraction of muscle fibers |
myosin head binds to actin causes shortening of muscle fiber pulls Z discs toward M line I bands shorten Z discs closer H zones disappear A bands move closer
|
|
|
2 things in order for skeletal muscle to contract |
activation (at neuromuscular junction) excitation-contraction coupling |
|
|
for skeletal muscle to contract: activation |
at neuromuscular junction must be nervous system stimulation must generate action potential in sarcolemma |
|
|
for skeletal muscle to contract: excitation-contraction coupling |
action potential propagated along sarcolemma intracellular calcium levels must rise briefly |
|
|
skeletal muscles stimulated by |
somatic motor neurons |
|
|
each axon ending forms what with single muscle fiber |
neuromuscular junction |
|
|
synaptic cleft |
gel-filled space that separates axon terminal of neuromuscular junction and muscle fiber |
|
|
synaptic vesicles of axon terminal contain neurotransmitter |
acetylcholine (ACh) |
|
|
junctional folds of sarcolemma contain |
ACh receptors |
|
|
neuromuscular junction contains what three things |
axon terminals synaptic cleft junctional folds |
|
|
events at the neuromuscular junction |
nerve impulse arrives at axon terminal----> ACh released into synaptic cleft ACh diffuses across cleft and binds with receptors on sarcolemma electrical events----> generation of action potential |
|
|
Acetylcholine effects quickly terminated by what enzyme in synaptic cleft |
acetylcholinesterase |
|
|
effect of acetylcholinesterase on ACh |
breaks down ACh to acetic acid and choline prevents continued muscle fiber contraction in absence of additional stimulation |
|
|
generation of action potential |
resting sarcolemma polarize----> voltage across membrane action potential caused by changes in electrical charges occurs in three steps |
|
|
three steps that action potential occurs in |
end plate potential depolarization repolarization |
|
|
generation of action potential: end plate potential (local depolarization) |
ACh binding opens chemically (ligand) gated ion channels simultaneous diffusion of sodium inward and potassium outward more sodium diffuses in, so interior of sarcolemma becomes less negative |
|
|
local depolarization= |
end plate potential |
|
|
generation of action potential: depolarization |
end plate potential spreads to adjacent membrane areas voltage-gated sodium channels open sodium influx decreases membrane voltage toward critical voltage called threshold if threshold reached, AP initiated once initiated, is unstoppable---> muscle fiber contraction |
|
|
depolarization |
generation and propagation of an action potential (AP) |
|
|
generation of action potential: repolarization |
sodium channels close and voltage-gated potassium channels open potassium efflux rapidly restores resting polarity fiber cannot be stimulated (in refractory period until repolarization complete) ionic conditions of resting state restored by sodium potassium pump |
|
|
repolarization |
restoring electrical conditions of RMP |
|
|
excitation-contraction (E-C) coupling main points |
events that transmit AP along sarcolemma lead to sliding of myofilaments AP brief, ends before contraction latent period |
|
|
excitation-contraction (E-C) coupling causes rise in intracellular ______ which causes contraction |
calcium |
|
|
latent period of excitation-contraction (E-C) coupling |
time when E-C coupling events occur time between AP initiation and beginning of contraction |
|
|
events of excitation-contraction (E-C) coupling |
AP propagated along sacromere to T tubules voltage-sensitive proteins stimulate calcium release from SR calcium necessary for contraction |
|
|
role of calcium in contraction: at low intracellular calcium concentration |
tropomyosin blocks active sites on actin myosin heads cannot attach to actin muscle fiber relaxed |
|
|
what blocks active sites on actin |
tropomyosin |
|
|
role of calcium in contraction: at higher intracellular calcium concentrations |
calcium binds to troponin troponin changes shape and moves tropomyosin away from myosin-bidning sites myosin heads bind to actin, causing sacromere shortening and muscle contraction when nervous stimulation ceases, calcium pumped back into SR and contraction ends |
|
|
cross bridge cycle continues as long as |
calcium signal and adequate ATP present |
|
|
4 steps of cross bridge cycle |
1. cross bridge formation 2. working (power) stroke 3. cross bridge detachment 4. cocking of myosin head |
|
|
first step of cross bridge cycle |
cross bridge formation high-energy myosin head attaches to thin filament |
|
|
2nd step of cross bridge cycle |
working (power) stroke myosin head pivots and pulls thin filament toward M line |
|
|
3rd step of cross bridge cycle |
cross bridge detachment ATP attaches to myosin head and cross bridge detaches |
|
|
4th step of cross bridge cycle |
cocking of myosin head energy from hydrolysis of ATP cocks myosin head into high-energy state |
|
|
isometric contraction |
no shortening muscle tension increases but does not exceed load |
|
|
isotonic contraction |
muscle shortens because muscle tension exceeds load |
|
|
force and duration of a muscle contraction vary in response to |
stimuli of different frequencies and intensities |
|
|
motor nerves |
each muscle served by at least one motor nerve motor nerve contains axons of up to hundreds of motor neurons axons branch into terminals-----> NMJ with single muscle fiber |
|
|
motor unit |
motor neuron and all (4 to several hundred) muscle fibers it supplies (smaller number= fine control) |
|
|
muscle fibers from motor unit spread thoughout muscle so single motor unit causes |
weak contraction of entire muscle usually contract synchronously-----> helps prevent fatigue |
|
|
muscle twitch |
motor unit's response to single AP of its motor neuron simplest contraction observable in lab (recorded as myogram) muscle contracts faster than it relaxes |
|
|
three phases of muscle twitch |
latent period period of contraction period of relaxation |
|
|
latent period of muscle twitch |
events of excitation-contraction coupling no muscle tension |
|
|
period of contraction of muscle twitch |
cross bridge formation tension increases |
|
|
period of relaxation of muscle twitch |
calcium reentry into SR tension declines to zero |
|
|
different strength and duration of twitches due to variations in |
metabolic properties and enzymes between muscles |
|
|
muscle twitch only occurs in... |
lab or neuromuscular problems |
|
|
characteristics of isotonic contractions |
muscle changes in length and moves load (thin filaments slide) either concentric or eccentric |
|
|
2 types of isotonic contractions |
concentric contractions eccentric contractions |
|
|
concentric contraction |
type of isotonic contraction muscle shortens and does work |
|
|
eccentric contractions |
type of isotonic contraction muscle generates force as it lengthens |
|
|
characteristics of isometric contractions |
load greater than tension muscle can develop tension increases to muscle's capacity, but muscle neither shortens or lengthens |
|
|
activity of cross bridges during isometric contractions |
cross bridges generate force but do not move actin filaments |
|
|
characteristics of muscle tone |
constant, slightly contracted state of all muscles due to spinal reflexes keeps muscle firm, healthy and ready to respond |
|
|
how is muscle tone due to spinal reflexes |
groups of motor units alternately activated in response to input from stretch receptors in muscles |
|
|
energy for muscle contractions |
ATP only source used directly for contractile activities available stores of ATP depleted in 4-6 seconds |
|
|
how is ATP used in contraction of muscle |
move and detach cross bridges calcium pumps in SR return of sodium and potassium after excitation-contraction coupling |
|
|
in muscle contraction, ATP is regenerated by |
direct phosphorylation of ADP by creatine phosphate (CP) anaerobic pathway (glycolysis----> lactic acid) aerobic respiration |
|
|
characteristics of anaerobic pathway |
glycolysis does not require oxygen at 70% of maximum contractile activity lactic acid |
|
|
glycolysis during anaerobic pathway |
glucose degraded to 2 pyruvic acid molecules normally enter mitochondria----> aerobic respiration bulging muscles compress blood vessels---> oxygen delivery impaired pyruvic acid converted to lactic acid |
|
|
lactic acid |
anaerobic pathway diffuses into bloodstream used as fuel by liver, kidneys and heart converted back into pyruvic acid or glucose by liver anaerobic respiration yields only 5% as much ATP as aerobic respiration, but produces ATP 2 and a half times faster |
|
|
characteristics of aerobic respiration |
produces 95% of ATP during rest and light to moderate exercise---> slow series of chemical reactions that require oxygen---> occur in mitochondria |
|
|
chemical reaction that occur in mitochondria during aerobic respiration |
breaks glucose into CO2, H2O, and large amounts of ATP |
|
|
fuels of aerobic respiration |
stored glycogen, then bloodborne glucose, pyruvic acid from glycolysis, and free fatty acids |
|
|
2 energy systems during sports |
aerobic endurance anaerobic threshold |
|
|
aerobic endurance |
length of time muscle contracts using aerobic pathways |
|
|
anaerobic threshold |
point at which muscle metabolism converts to anaerobic |
|
|
force of muscle contraction depends on |
number of cross bridges attached |
|
|
the number of cross bridges attached during contraction is affected by what 4 things |
1. number of muscle fibers stimulated 2. relative size of fibers: hypertophy of cells increases strength 3. frequency of stimulation 4. degree of muscle stretch |
|
|
muscle fiber type is classified according to what 2 characteristics |
speed of contraction metabolic pathways for ATP synthesis |
|
|
muscle fiber classified according to speed of contraction |
slow or fast fibers according to: speed at which myosin ATPases split ATP pattern of electrical activity of motor neurons |
|
|
muscle fiber classification according to metabolic pathways for ATP synthesis |
oxidative fibers: use aerobic pathways glycolytic fibers: use anaerobic glycolysis |
|
|
oxidative fibers |
use aerobic pathways |
|
|
glycolytic fibers |
use anaerobic glycolysis |
|
|
three types of muscle fibers |
slow oxidative fibers fast oxidative fibers fast glycolytic fibers |
|
|
aerobic (endurance) exercise leads to increased (3) |
muscle capillaries number of mitochondria myoglobin synthesis |
|
|
aerobic (endurance) exercise results in |
greater endurance, strength and resistance to fatigue may convert fast glycolytic fibers into fast oxidative fibers |
|
|
results of resistance exercise (typically anaerobic) |
muscle hypertrophy (due primarily to increase in fiber size) increased mitochondria, myofilaments, glycogen stores and connective tissue increased muscle strength and size |
|
|
homeostatic imbalance of muscles |
rigor mortis |
|
|
rigor mortis |
homeostatic imbalance of muscle cross bridge detachment requires ATP 3-4 hours after death muscles begin to stiffen with weak rigidity at 12 hours post mortem |
|
|
what 4 things determines the muscle contraction |
1. length of sacromere at time of contraction 2. speed of sacromere contraction 3. sacromere arrangment 4. arrangment of sacromeres acroos entire muscle |
|
|
under normal conditions a sacromere will shorten no more than |
1 micrometer |
|
|
low velocity of sarcomere contraction= |
high force
|
|
|
high velocity of sarcomere contraction= |
low force |
|
|
the greater the number of sacromeres in a line, the _____ the total amount of muscle shortening possible |
greater |
|
|
the greater number of sarcomeres in a line, the ______ the contraction velocity |
higher |
|
|
muscle force diminishes as contraction velocity _____ |
increases |
|
|
parallel arrangement of sarcomeres |
the higher the # of sarcomeres parallel, the greater the force |
|
|
serial arrangement of sarcomeres |
greater the # of sarcomeres in a line the greater the degree of shortening (how quickly)
|
|
|
longest muscle in body is how many micrometers |
100,000 |
|
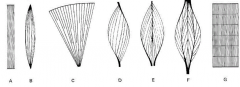
a |
straplike muscle |
|
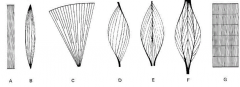
B |
spindle-shaped muscle biceps and triceps |
|
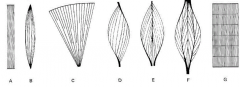
C |
fan-shaped muscle pecs very powerful in early contraction |
|
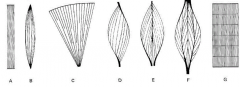
D |
pennate muscle |
|
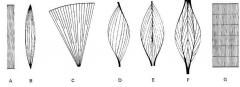
E |
bipennate muscle |
|
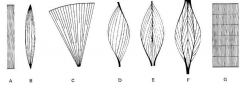
F |
multipennate muscle |
|
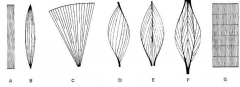
G |
segmented muscle |
|
|
what three arrangements of muscles shorten by less than one micrometer and hold things in place |
pennate muscle bipennate muscle multipennate muscle |
|
|
gastrocnemius muscle |
behind knee by calf |
|
|
the velocity will _____ the more sacromeres are contracted at once (in line) |
increase |
|
|
within skeletal muscle, not all fibers may be |
stimulated during the same interval |
|
|
graded responses |
different degrees of skeletal muscle shortening 4 types: twitch tetanus unfused tetanus fused tetanus |
|
|
4 types of grades responses |
twitch tetanus unfused tetanus fused tetanus
|
|
|
graded response- twitch |
single, brief contraction not a normal muscle function |
|
|
graded response- tetanus
|
(summing of contractions) one contraction is immediately followed by another muscle does NOT completely return to a resting state the effects are added |
|
|
graded response- unfused (incomplete) tetanus |
some relaxation occurs between contractions the results are summed |
|
|
graded response- fused (complete) tetanus |
no evidence of relaxation before the following contractions the result is a sustained muscle contraction |
|
|
muscle force depends upon the number of |
fibers stimulated |
|
|
more fibers contracting results in |
greater muscle tension |
|
|
muscles can continue to contract unless they |
run out of energy |
|
|
direct phosphorylation |
(energy for muscle contraction) muscle cells contain creatine phosphate (CP) after ATP is depleted, ADP is left CP transfers energy to ADP to regenerate ATP
|
|
|
CP (creatine phosphate) supplies during direct phosphorylation are exhausted in about |
20 seconds |
|
|
aerobic respiration occurs in the |
mitochondria |
|
|
during aerobic respiration, glucose is broken down into |
carbon dioxide, water and releasing energy |
|
|
aerobic respiration creates ____ muscle fibers |
red |
|
|
lactic acid produces muscle |
fatigue |
|
|
anaerobic glycolysis produces ____ muscle fibers |
white |
|
|
when a muscle is fatigued, it is unable to |
contract |
|
|
the common reason for muscle fatigue is |
oxygen debt oxygen must be repaid to tissue to remove oxygen debt oxygen is required to get rid of accumulated lactic acid |
|
|
increasing acidity (lactic acid) and lack of ATP cause the muscle to |
contract less |
|
|
different fibers contract at different times to provide |
muscle tone |
|
|
the process of stimulating various fibers is under ______ control |
involuntary |
|
|
4 results of increased muscle use |
1. increase in muscle size 2. increase in muscle strength 3. increase in muscle efficiency 4. muscle becomes more fatigue resistant |
|
|
movement of muscle is attained due to a muscle contracting and |
moving an attached bone |
|
|
prime mover muscle |
muscle with the major responsibility for a certain movement |
|
|
antagonist muscle |
muscle that opposes or reverses a prime mover |
|
|
synergist muscle |
muscle that aids a prime mover in a movement and helps prevent rotation |
|
|
fixator muscle |
stabilizes the origin of a prime mover |
|
|
7 categories of naming skeletal muscles |
1. direction of muscle fibers 2. relative size of the muscle 3. location of muscle 4. number of origins 5. location of muscles origin and insertion 6. shape of muscle 7. action of the muscle |
|
|
naming skeletal muscles: example of direction of muscle fibers |
rectus (straight) |
|
|
naming skeletal muscles: example of relative size of muscle |
maximus (largest) |
|
|
naming skeletal muscles: example of location of muscle |
many muscles named for bones temporalis |
|
|
naming skeletal muscles: example of number of origins |
triceps (three heads) |
|
|
naming skeletal muscles: example of location of the muscles origin and insertion |
sterno (on the sternum) |
|
|
naming skeletal muscles: example of shape of muscle |
deltoid (triangular) |
|
|
naming skeletal muscles: example of action of the muscle |
flexor and extensor (flexes or extends a bone) |
|
|
articulation |
also called joint forms the connection between two or more bony structures |
|
|
2 ways articulations are classified |
functionally structurally |
|
|
3 functional classifications of articulations |
synarthroses amphiarthroses diathroses s |
|
|
synarthroses |
functional classification means "together" immovable articulations these connections designed to improve the overall strength of bony structure (skull) |
|
|
amphiarthroses |
functional classification slightly movable articulation will "give" under extreme conditions (pelvic symphysis during birthing) |
|
|
diarthroses |
functional classification freely movable articulations structures usually thought of as "joints" (knee, shoulder articulations) |
|
|
3 structural classifications of articulations |
fibrous articulations cartilaginous articulations synovial articulations |
|
|
fibrous articulations |
generally synarthrotic and relatively simple connections bones united by fibrous TISSUE |
|
|
examples of fibrous articulations |
sutures syndesmoses: allows more movement than sutures (distal end of tibia and fibula) |
|
|
sutures connect the bones of the |
human skull to create a strong and protective vessel for the brain and special senses |
|
|
cartilaginous articulations |
structural classification cushioning syn- to amphiarthrotic articulations bones connected by fibro cartilage
|
|
|
examples of cartilaginous articulations |
pubic symphysis intervertebral articulations |
|
|
synovial articulations |
structural classification diathrotic and often highly complex articulating bones separated by articulation cavity synovial fluid is found in articulation cavitye |
|
|
example of synovial plane joint |
carpals |
|
|
example of synovial hinge joint |
humerus |
|
|
example of synovial pivot joint |
ulna and radius |
|
|
example of synovial condyloid joint |
metacarpal phalanx |
|
|
example of synovial saddle joint |
carpal metacarpal #1 |
|
|
example of synovial ball and socket joint
|
head of humerus scapula |
|
|
3 inflammatory conditions associated with joints |
bursitis tendonitis arthritis |
|
|
bursitis |
inflammatory condition of joint inflammation of bursa usually caused by blow or friction |
|
|
tendonitis |
inflammatory condition of joint inflammation of tendon sheaths |
|
|
arthritis |
inflammatory or degenerative diseases of joints over 100 different kinds the most widespread crippling disease in the US |
|
|
3 clinical forms of arthritis |
osteoarthritis rheumatoid arthritis gouty arthritis |
|
|
osteoarthritis |
most common chronic arthritis probably related to normal aging processes |
|
|
rheumatoid arthritis |
an autoimmune disease (immune system attacks joints) symptoms begin with bilateral inflammation of certain joints often leads to deformities |
|
|
gouty arthritis |
inflammation of joints is caused by a deposition of urate crystals from the blood can usually be controlled with diet |
|
|
functions of trunk muscles |
support trunk posture aid vetilation support abdominal organs attachment for movers of the brachium |
|
|
functions of deep abdominal muscles |
ventilation abdominal organ support |
|
|
arrangement of abdomincal aponeuroses and musculature to achieve |
great structural strength in all directions |
|
|
function of posteior trunk muscles |
aid upright posture support lumbar region provide abdominal protection attachment site for brachial musculature |
|
|
function of the muscles of the brachium |
locomotion prime movers tool use heat production |
|
|
functions of the muscles of the pelvis, hip and thigh |
support of upright posture locomotion heat production |
|
|
function of the muscles of the lower leg |
locomotion muscular weight is carried closed to body |

