![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
280 Cards in this Set
- Front
- Back
|
Possible consequences of major renal failure
|
i. Metabolic Acidosis
ii. Hyperkalemia iii. Uremic Toxicity iv. Na+/H2O Imbalance v. Ca2+/PO42- Imbalance vi. Plasma Protein vii. Anemia viii. Depressed Immune System |
|
|
Azotemia in kidney
|
↑ plasma creatinine and BUN
|
|
|
Anemia in kidney
|
↓ erythropoietin synthesis
|
|
|
Acute renal failure (ARF)
|
typically reversible
3 Types of ARF: Pre-renal ARF, Intra-renal ARF, Post-renal ARF |
|
|
Pre-renal ARF
|
↓ renal blood flow --> ↓ glomerular filtration rate
|
|
|
Intra-renal ARF
|
acute tubular necrosis (ATN) - ischemia/toxin-induced
ATN will have sloughing off of cells |
|
|
Post-renal ARF
|
Urinary tract obstruction
Kidney stones are a good example |
|
|
Chronic renal failure (CRF)
|
Irreversible, usually progressive renal injury
Causes: diabetes (34%) hypertension (29%) glomerulonephritis (14%) |
|
|
End-stage renal disease (ESRD)
|
GFR < 10% of normal
Approximately 500,000 people/year treated for ESRD |
|
|
Renal failure (K+ and HCO3-)
|
↑ plasma K+ concentration (hyperkalemia)
↓ plasma HCO3- concentration (metabolic acidosis) |
|
|
Hemodialysis
|
Blood pumped through an external artificial dialyzer
Typically three times per week, each lasting 3-4 hours |
|
|
Peritoneal dialysis
|
Fluid in the peritoneal cavity; peritoneum acts as dialysis membrane
Chronic ambulatory peritoneal dialysis (CAPD); fluid exchanged 4-6 times per day Higher risk of infection than with hemodialysis |
|
|
Limitation of intermittent hemodialysis
|
Body fluid homeostais cannot be maintained
Between sessions: Body weight increases due to retention of water (input > output) Plasma creatinine increases (synthesis > output) Kidneys “process” plasma continuously (60X/day), minimizing composition changes |
|
|
π = nCRT
|
n = number of dissociable particles/molecule
C = total solute concentration R = gas constant T = temperature in degrees Kelvin |
|
|
OsmolaRity
|
# of solute particles per liter of solvent (temperature dependent)
|
|
|
OsmolaLity
|
# of solute particles per kilogram of solvent (temperature independent)
|
|
|
Total body water (TBW)
|
60% of body weight; affected by percent body fat (Increase fat = Decrease H2O)
TBW is highest in newborns and adult males and TBW is lowest in adult females and in adults with a large amount of adipose tissue |
|
|
Intracellular fluid (ICF)
|
2/3 of TBW
Major cations: K+ and Mg2+ Major anions: protein and organic phosphates |
|
|
Extracellular fluid (ECF)
|
1/3 of TBW
Major cations: Na+ Major anions: Cl- and HCO3- |
|
|
Plasma
|
1/4 of ECF
1/12 of TBW Major proteins: albumin and globulins |
|
|
Interstitial fluid
|
3/4 of ECF
1/4 TBW Little protein Ultrafiltrate of plasma |
|
|
60-40-20 Rule
|
TWB is 60% of body weight
ICF is 40% of body weight ECF is 20% of body weight |
|
|
Blood volume
|
Plasma volume (ECF) + red blood cell volume (ICF)
|
|
|
Fluid Volume (equation)
|
Amount of X Added /
Concentration of X at Equilibrium |
|
|
Compartment Volume (equation)
|
(Amount of X Given) - (Amount of X Lost) /
Concentration of X at Equilibrium |
|
|
A 1 mM solution of glucose exerts an osmotic pressure of what?
|
2.54 X 10-2 atm = 19.3 mmHg
|
|
|
What is the osmolarity of the ECF and ICF?
|
~ 300 mOsm/L
|
|
|
Markers: Extracellular Volume
|
Radiolabeled Sodium
Sucrose Mannitol Insulin |
|
|
Markers: Plasma Volume
|
Iodinated albumin
T-1842 (Evans Blue) |
|
|
Markers: Total Body Water
|
Antipyrine
Titrated water Heavy water |
|
|
Indirect: Interstitial Volume
|
Extracellular Fluid Volume - Plasma Volume
|
|
|
Indirect: Intracellular Volume
|
Total Body Water - Extracellular Fluid Volume
|
|
|
JV = KF [(PC – PI) – (πC – πI)]
|
JV = flux of fluid across the capillary wall (volume/time)
KF = the filtration coefficient PC = hydrostatic pressure within the capillary PI = hydrostatic pressure within the interstitium (tissue “turgor” pressure) πC = oncotic pressure within the capillary (osmotic pressure due to protein) πI = oncotic pressure within the interstitium |
|
|
Localized edema due to changes in one or more variables of Starling forces
|
↑ PC and Kf (e.g. due to inflammation)
↑ πI due to lymphatic obstruction preventing return of protein to the systemic circulation ↑ PC due to venous obstruction |
|
|
Generalized edema
|
Involves Na+ retention and expansion of the entire extracellular fluid volume
|
|
|
Disruption of normal intracellular-extracellular fluid volume (and osmolality) can be caused by?
|
ingestion of water
dehydration I.V infusions fluid losses |
|
|
How do you calculate changes in volume and osmolality?
|
At equilibrium, osmolalities of extra- and intracellular fluid must be equal
Shifts result from water movement only Always calculate the effect on total body water first |
|
|
Renal pyramids
|
Comprised of medullary tissue
Base originates at corticomedullary border Apex terminates in a papilla |
|
|
Nephron
|
1 million nephrons per adult kidney; each nephron one epithelial cell layer thick
Proximal tubule --> loop of Henle --> distal tubule --> collecting tubule |
|
|
Cortical Nephron
|
Glomerulus located close to the surface of the kidney
“short-looped” nephrons (no thin ascending limb) |
|
|
Juxtamedullary Nephron
|
Glomerulus located close to the border between cortex and medulla
“long-looped” nephrons (essential for urine concentration) |
|
|
Nephron Composition in Human Kidney
|
80% cortical
20% juxtamedullary nephrons |
|
|
Cortical/juxtamedullary nephron ratio
|
Cortical/juxtamedullary nephron ratio correlates with capacity to concentrate urine
(↑ percent juxtamedullary = ↑ concentrating ability) |
|
|
Renal Vasculature
|
Afferent arteriole --> Glomerular capillary --> Efferent arteriole --> Peritubular capillary
Allows resistance upstream and downstream of the glomerular capillaries to be regulated (critical for regulation of glomerular filtration) |
|
|
Vasa Recta
|
A subset of peritubular capillaries originating from efferent arterioles of juxtamedullary nephrons
“Hairpin-loop” orientation of the vasa recta parallel loops of Henle (play a critical role in maintaining the hypertonicity of the renal medulla) |
|
|
What % of CO goes to the kidneys?
|
25% of cardiac output (1.2-1.5 L/min)
|
|
|
The renal arterio-venous (a-v) O2 concentration difference is relatively low. This suggests that renal O2 consumption is low. However, O2 consumption of the kidneys is very high. Explain this paradox.
|
This paradox is explained by the fact that since blood flow to the kidneys is high, only a relatively low extraction % is required to provide O2 needs
(O2 consumption = a-v O2 difference X blood flow rate) |
|
|
Tubular secretion
|
Movement of a substance from the peritubular capillary into the lumen
Secretion mostly restricted to solutes that are poorly filtered due to size, charge or protein binding |
|
|
Tubular reabsorption
|
Movement from lumen into the peritubular capillary
This is the principal mechanism for modifying the composition of the filtered fluid |
|
|
Glomerular filtration
|
Filtration of plasma from glomerular capillaries into Bowman’s space
Only 20% of plasma entering the glomerular capillaries is filtered (the “filtration fraction”) |
|
|
Filtration fraction
|
= glomerular filtration rate (GFR) ÷ renal plasma flow rate (RPF)
|
|
|
Amount excreted =
(Renal filtering) |
Amount filtered (+ amount secreted) – amount reabsorbed
|
|
|
How many times a day is plasma filtered?
|
60X
If GFR = 180 L/day and plasma volume = 3 L then plasma volume is filtered 60X per day |
|
|
Urine Composition
|
Urine flow rate must be known to draw conclusions about excretory capacity
Presence of glucose, amino acids etc. in the urine suggestive of impaired renal function |
|
|
Urine flow to the bladder
|
Walls of calices, pelvis and ureters contain smooth muscle
Inherent pacemaker activity in calices initiates peristaltic contraction |
|
|
Micturition reflex
|
Pparasympathetic control
As the bladder fills with urine: ↑ stretch --> ↑ parasympathetic activity --> ↑ contraction of the detrusor muscle of the bladder ↓ skeletal motor neuron input (pudendal nerve) --> relaxation (opening) of the external sphincter |
|
|
Where can the micturition reflex be modulated?
|
Higher centers in the brain stem (pons)
Cerebral cortex |
|
|
Voluntary urination
|
Abdominal muscle contraction -->
↑ pressure in bladder --> ↑ stretch --> ↑ micturition reflex |
|
|
Atonic bladder
|
Loss of sensory nerve fibers
No micturition reflex therefore bladder overflows a few drops at a time (overflow incontinence) |
|
|
Automatic bladder
|
Spinal cord damage above the sacral region
Loss of higher center control (particularly suppression) of the micturition reflex (periodic unintended bladder emptying) |
|
|
Kidney Structure
|
Cortex = outer mass
Medulla = divided into pyramids deep in kidney Surround by minor calayx Minor -->major calyx -->renal pelvis |
|
|
Renal pelvis
|
Holding chamber for urine
|
|
|
There is a brush border on the proximal tubule.
TRUE or FALSE |
TRUE
|
|
|
Renal Artery
|
Breaks up to segmental artery
Segmental artery gives blood to a certain zone of the kidney Ligation the segmental arteries can nephrectomize a kidney --> used to reduce renal function (as seen in experiment) |
|
|
Importance of no venules within the glomerular capillaries
|
Arterioles constriction can be varied
Because you have arteriole both down and upstream from glomerulus, you can modulate hydrostatic pressure in glomerular capillaries very precisely --> allows you to regulate the GFR |
|
|
Difference in hematocrit between the afferent and efferent arteriole
|
Hematocrit is about 20% higher in efferent arteriole compared to afferent arteriole
|
|
|
Reabsorbed
|
Prevent it from being excreted by returning it to the body
|
|
|
What is the principal mechanism for modifying the composition of the filtered fluid?
|
Tubular reabsorption
|
|
|
Why do you reabsorb 50% of urea?
|
The reabsorbed urea does not go back into general circulation but stays in kidney to help water balance and concentrate urine
(kidneys can handle a high level of toxic material all with the goal to maintain water balance) |
|
|
Percentages of filtered load of reabsorbed not constant.
TRUE or FALSE |
TRUE
Changes based on state of body = vary the rate of absorption by changing flow rate depending on condition of body |
|
|
Dehydrated
|
Up to 1200 mOsm/kg H2O
|
|
|
Overhydrated
|
Down to 50-75 mOsm/kg
|
|
|
Urine Composition:
Na+ pH Osmolarity |
50 - 120 mEq/L
5-7 500 - 800 mOsm/kg |
|
|
Protein in Urine
|
There should be no protien in the urine composition. Protein in urine is tell-tale sign of kidney dysfunction.
|
|
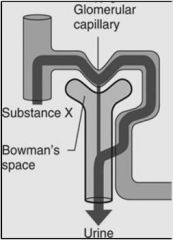
|
Example: (PAH)
Something that is this heavily excreted can be used to measure renal plasma flow |
|
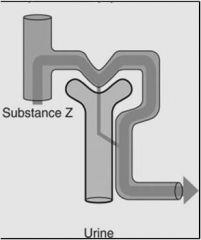
|
Example: (Glucose, bicarbonate)
|
|
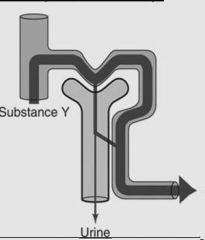
|
Example: (NaCl, water, calcium)
|
|
|
Why is there glucose in the urine during diabetes?
|
Kidney is not really trying to restore homeostasis by excreting glucose. It is still working its hardest to reabsorb glucose. But the plasma has so much glucose in a diabetic that it can’t reabsorb everything and so you see glucose in your urine.
|
|
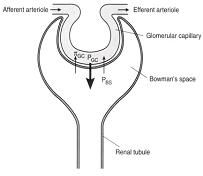
JV (GFR) = KF [(PGC – PBS) – πGC ]
|
PGC = glomerular capillary hydrostatic pressure
PBS = bowman’s space hydrostatic pressure πGC = glomerular capillary oncotic pressure KF = ultrafiltration coefficient |
|
|
The glomerular capillaries contained in a proximal tubule extension known as what?
|
Bowman's Capsule
|
|
|
The “filtration barrier”
|
endothelium (fenestrated)
↓ basement membrane (collagen, laminin, fibronectin) ↓ epithelial podocytes (foot processes encircle outer surface of capillaries; connected by slit membranes) |
|
|
Mesangial cells
|
Provide structural support
Secrete extracellular matrix Possess phagocytic activity Secrete prostaglandins / cytokines Possess contractile activity |
|
|
Formation of an "Ultrafiltrate of Plasma"
|
Solutes with a molecular weight < 5000 are freely filtered (thus the concentration of solutes such as NaCl and glucose filtered into Bowman’s space will be the same as in plasma)
Above 5000 "filterability" governed by: Size Charge |
|
|
Effect of charge in Ultrafiltrate
|
High glycoprotein content of basement membrane (and endo- and epithelial cells) imparts net negative charge to the "filtration pathway"
Charge selectivity often lost in glomerular diseases (nephrotoxic serum nephritis) |
|
|
Glomerular capillaries in contrast to systemic capillaries
|
Hydrostatic pressure remains relatively constant along the length of the glomerular capillary (due to presence of resistance points before and after the glomerular capillaries)
πGC progressively increases along the capillary because as fluid is filtered out the concentration of the non-filterable proteins increases |
|
|
Net Filtration Pressure
|
= 0 when πGC increases to the point that it = [PGC - PBS]
|
|
|
Epithelial podocytes (foot processes)
|
Foot processes encircle outer surface of capillaries; connected by slit membranes
|
|
|
The Ultrafiltration Coefficient (KF)
|
NFP (net filtration pressure) similar in glomerular and systemic capillaries (10-20 mmHg)
But the volume of fluid filtered across glomerular capillaries (180 L/day) is much greater than across systemic capillaries (approx 4L/day) because Glomerular capillary ultrafiltration coefficient (KF) is much higher than in systemic capillaries Changes in KF can affect filtration dynamics (primarily by affecting the rate of change of πGC) e.g. ↑ KF --> ↑ rate of rise of πGC --> NFP=0 at an earlier point along the capillary |
|
|
Why is the volume of fluid filtered across glomerular capillaries (180 L/day) much greater than across systemic capillaries?
|
Because Glomerular capillary ultrafiltration coefficient (KF) is much higher than in systemic capillaries
KF = LP x A LP = hydraulic permeability: (100X higher than systemic capillaries) A = capillary surface area: (2X higher than skeletal muscle) |
|
|
GFR regulated primarily by regulating glomerular capillary hydrostatic pressure (PGC)
|
Fundamentally an ↑ PGC = ↑ GFR and vice versa
Alterations in either afferent or efferent arteriolar resistance can affect PGC Note the effects of afferent and efferent resistance changes on renal plasma flow (RPF) compared to effects on GFR |
|
|
What two vasoconstrictors are primarily responsible for increasing the resistance (diameter) of the afferent arteriole?
|
Renal Sympathetic Nerves
(vasoconstrictor = ↓ GFR) Angiotensin II (vasoconstrictor) |
|
|
The Modulatory Role of Vasodilatory Prostaglandins
|
Major renal vasodilatory prostaglandins = PGE2 and PGI2 (prostacyclin)
↑ renal sympathetic activity (RSN) and/or ↑ AII → ↑ PGE2 and PGI2 synthesis RSN / AII plus PGE2 / PGI2 results in lesser degree of afferent vasoconstriction that RSN / AII alone |
|
|
Pathology: Changes in the Ultrafiltration Coefficient (KF)
|
Glomerular disease (affecting Lp and/or A)
Mesangial cell contractility (altering A) |
|
|
Pathology: Changes in capillary oncotic pressure
|
Changes in systemic plasma protein concentration (liver disease)
|
|
|
Pathology: Changes in intratubular pressure
|
Ureteral obstruction (ureteral stone)
The ureterorenal reflex: UO → reflex constriction → sympathetic reflex → renal arteriole constriction → ↓ GFR |
|
|
The ureterorenal reflex
|
UO → reflex constriction → sympathetic reflex → renal arteriole constriction → ↓ GFR
|
|
|
If PGC is the primary determinant of glomerular filtration, it would be anticipated that GFR will constantly change with the normal fluctuations in blood pressure that occur
TRUE or FALSE |
FALSE
This does NOT occur because the kidneys AUTOREGULATE |
|
|
Autoregulation of Kidneys
|
Intrinsic ability of the kidney to maintain GFR (and renal plasma flow; RPF) constant over wide range of blood pressure (~ 70 mmHg and above)
|
|
|
Must be an intrinsic (intrarenal) mechanism because autoregulation is maintained in (2)?
|
Transplanted kidneys (therefore cannot be a central neural mechanism)
Isolated perfused kidneys (an in vitro preparation devoid of hormonal inputs) |
|
|
Control site of kidney autoregulation
|
Afferent arteriole (resistance changes cause parallel GFR and RPF changes)
|
|
|
Proposed Mechanisms of Autoregulation (2)
|
Myogenic Theory
Tubuloglomerular Feedback Theory |
|
|
Myogenic Theory
|
Reflex resistance changes in the afferent arteriole
(e.g. ↑ b.p. → ↓ a.a. diameter → minimal ↑ in PGC) |
|
|
Tubuloglomerular Feedback Theory
|
A change in flow rate or composition of tubular fluid sensed at the macula densa results in a compensatory change in GFR (and RPF)
|
|
|
What is the sensor in the Tubuloglomerular Feedback Theory?
|
Macula Densa
|
|
|
Importance of Autoregulation
|
Blunts large changes in water and solute excretion that would otherwise occur whenever arterial pressure changed
|
|
|
Limits of Autoregulation
|
Not perfect (GFR and RPF do change slightly)
Does not occur below b.p. of 70 mmHg CAN BE OVERRIDDEN |
|
|
Filterability Scale
|
1 means no problem
The lower the number harder it is to filter |
|
|
Nephrotoxic Serum Nephritis (NSN) effect on filterability
|
NSN messes up the structure of the filtration membrane, causing it to loose its negative charge --> charge selectivity is gone
|
|
|
What would be the difference in protein concentration at the NFP point when it equals 0 in the glomerular capillary?
|
Higher (20%)
|
|
|
Pathology can change the structure of the glomerular capillaries:
change the Lp (water permeability) Increase Kf Decrease Kf |
change the Lp (water permiability) -->
change the Kf (ultrafiltration coefficient) Increase Kf --> Volume of fluid filtered is still the same, just took less of the capillaries to filter Decrease Kf --> longer rate of filtration --> never get to equilibrium --> total volume of fluid filtered will be less (will reduce GFR) |
|
|
Substances can be transported in one of two directions
|
Lumen --> Peritubular capillary = TUBULAR REABSORPTION
Peritubular capillary --> Lumen = TUBULAR SECRETION |
|
|
Tubular Reabsorption
|
Lumen --> Peritubular capillary = TUBULAR REABSORPTION
|
|
|
Tubular Secretion
|
Peritubular capillary --> Lumen = TUBULAR SECRETION
|
|
|
Transport can occur across the renal epithelial cells in two ways:
|
Transcellular (transport)
Paracellular (transport) |
|
|
Transcellular (transport)
|
Through the cell across two membranes (luminal & basolateral)
|
|
|
Paracellular (transport)
|
Between cells (across tight junctions; typically by simple diffusion)
|
|
|
“Passive” transport across a membrane with a favorable electrochemical gradient via (2)
|
Channels
Carriers |
|
|
Carriers (3)
|
uniporters
symporters antiporters |
|
|
“Active” transport across a membrane against the electrochemical gradient via
|
Carrier-mediated “active transport” (e.g. Na+-K+-ATPase; Ca2+-ATPase)
|
|
|
Absolute dependence on Na+-K+-ATPase located ONLY on the basolateral membrane
TRUE or FALSE |
TRUE
Maintains low intracellular Na+ concentration |
|
|
Na+ in Cortical Collecting Tubule
|
Na+ enters the cell via luminal membrane Na+-selective ion channels
|
|
|
Na+ in Proximal Tubule
|
Na+ enters the cell via luminal membrane Na+-glucose co-transporters
“Downhill” Na+ movement across luminal membrane facilitates “uphill” glucose movement Glucose uniporter transports glucose out of the cell across the basolateral membrane |
|
|
Approximately two thirds of the glomerular filtrate is reabsorbed in the proximal tubule
|
180 L of water filtered/day → 120 L reabsorbed proximally/day
26,000 mEq of Na+ filtered/day → 17,000 mEq reabsorbed proximally/day Many solutes (glucose, amino acids, HCO3-) essentially completely reabsorbed proximally Note that chloride is reabsorbed (primarily passively) in the later section of the proximal tubule |
|
|
Isosmotic fluid reabsorption
|
Since 2/3rd of filtered water AND 2/3rd of the filtered solute is reabsorbed from the lumen of the proximal tubule the tubule fluid remains isotonic
|
|
|
If tubular fluid and interstitial fluid are both isotonic (~ 300 mOsm/kg), where is the required osmotic gradient for water reabsorption?
|
Step 1: solute transport creates a small transepithelial osmotic gradient (tubular fluid (TF) osmolality slightly lower than that of the extracellular (EC) fluid)
Step 2: water moves from lumen to interstitium down this osmotic gradient |
|
|
Complete reabsorption in the proximal tubule is a two-phase process
|
Movement (of water and solutes) from lumen to interstitium
Movement from interstitium into peritubular capillaries (dependent on STARLING FORCES) |
|
|
Peritubular capillary (PC) uptake promoted by what?
|
Low PC hydrostatic pressure (downstream of afferent / efferent arteriolar resistances)
High PC oncotic pressure (filtration creates high PC plasma protein concentration) |
|
|
Glomerulotubular (GT) Balance
|
The proximal tubule typically reabsorbs a constant percentage (~67%) of the filtered load
GT balance helps to maintain a relatively constant delivery of fluid to the distal nephron |
|
|
Consider the effects of an increase in efferent arteriolar resistance (constriction)
|
↑ resistance → ↑ PGC → ↑ GFR
BUT proximal tubule fluid reabsorption will also increase (GT balance) ↑ resistance → ↓ PPC, ↑ resistance → ↓ renal blood flow (RBF) → ↑ filtration fraction (GFR/RPF) → ↑ πPC |
|
|
Analysis of Renal Tubular Function using In Vivo Micropuncture
|
Pipette A samples tubular fluid at last accessible point of proximal tubule; difference in composition of this fluid and plasma represents transport along proximal tubule
Difference in composition of fluid sampled from pipettes A and B represents composite effects of late proximal tubule (pars recta) and segments of the loop of Henle Cannot assess collecting tubule or juxtamedullary nephron function by micropuncture (neither accessible from surface of the kidney) |
|
|
Tubular transport maximum (TM)
|
Since the reabsorption (and secretion) of most substances is channel or carrier-mediated, there must be a maximal transport capacity when all carriers are fully saturated.
|
|
|
Amount filtered (TM)
|
GFR x PGL
|
|
|
Amount excreted (TM)
|
V x UGL
|
|
|
Amount reabsorbed (TM)
|
Amt. filtered - Amt. excreted
|
|
|
“Splay”
|
Represents the slight variance in TM between individual nephrons
The splay is the curve in the reabsorbed line. Its not an immediate 90 degree turn because not all the nephrons have the same Tm and so the variance causes the transition to threshold to be not as immediate. |
|
|
“Threshold”
|
Represents the plasma concentration (of glucose) at which the TM is exceeded
Note that the normal plasma glucose concentration is well below threshold Threshold is not a fixed value (dependent on GFR) |
|
|
Diabetes mellitus (glucose and urine)
|
Diabetes mellitus is characterized by glucose in the urine (“glucosuria).
This is not a compensatory response of the kidneys to try and lower plasma glucose; the amount of glucose filtered >> TM |
|
|
Glucosuria
|
Glucose in the urine
|
|
|
Secretion in Proximal Tubule
|
Proximal tubule is the primary site of secretion for organic anions and cations.
Secretion essential for substances which must be excreted but are poorly filtered because of: i. molecular size or charge ii. plasma protein binding |
|
|
Transport of PAH into the cell across the basolateral membrane of the proximal tubule is dependent on how many transporters?
|
3
|
|
|
The α-ketoglutarate (αKG)-para-aminohippurate (PAH) antiporter is relatively non-selective.
TRUE or FALSE |
TRUE
a number of different organic anions can bind and be transported |
|
|
Requirements of substances used to measure GFR
|
Freely filtered by the glomerulus
Not reabsorbed or secreted by the nephron Not metabolized or produced by the kidney |
|
|
Examples of GFR markers
|
Inulin: Polyfructose; must be infused to achieve a steady-state plasma level
Creatinine: Breakdown product of creatine; plasma levels normally relatively stable |
|
|
PIN X GFR = V X UIN
|
PIN = plasma concentration of inulin
V = urine flow rate UIN = urine concentration of inulin |
|
|
The clearance of what substances is a measurement of GFR (2)?
|
Inulin
Creatinine |
|
|
Simultaneously measuring the clearance of substance X and the clearance of inulin or creatinine can provide an indication of how X is handled by the kidney
|
When the clearance of a substance is < CIN the substance must
When the clearance of a substance is > CIN the substance must undergo net secretion Do some substances have a clearance of zero? |
|
|
RPFa x PX = [RPFv x Px] + [Ux x V]
|
RPF = renal plasma flow
a = artery v = vein PX = plasma conc. of x UX = urine conc. of x V = urine flow rate |
|
|
At low plasma concentrations of PAH, how much will be filtered and/or secreted? How much will remain in the renal vein?
|
All PAH delivered to the kidney will be filtered and/or secreted
Thus [PAH] in the renal vein = 0 |
|
|
Renal Blood Flow (Equation)
|
RPF / (1 - Hematocrit)
|
|
|
What is the predominant route for chloride reabsorption?
|
The paracellular route
|
|
|
Where is most of the Cl reabsorbed?
|
Later part of the proximal tubule is where most of the Cl is reabsorbed
Water is leaving the tubule while the concentration of Cl is not changing --> tubular fluid conc. of Cl is increasing --> creates a conc. gradient for Cl transport |
|
|
The membrane in proximal tubules is very porus to water.
TRUE or FALSE |
TRUE
This is due to presence of aquaporins along the membrane Therefore a very small osmotic differential is enough to still move a large volume of water |
|
|
Hydrostatic pressure within the peritubular capillary major force opposing uptake
|
TRUE
Low hydrostatic pressure in capillaries is low since you are downstream 2 major resistance points |
|
|
Oncotic pressure is a major force promoting uptake in capillary
TRUE or FALSE |
TRUE
High oncotic pressure in capillaries due to filtration where a large amount of liquid and solute were filtered out, but the proteins were not --> rise in oncotic pressure |
|
|
Secretion in Proximal Tubule
|
Taking out of peritubuluar capillaries interstitium --> the tubular lumen
|
|
|
Secretion in Proximal Tubule
3 Step Process |
Get PAH into cell using PAH-alpha ketoglutarate antiporter
aKG transported back into the cell using the Na-aKG symporter (the low intracellular Na maintained by the Na-K-ATPase **rate limiting step still Na-K-ATPase Transported out to lumen using a PAH-anion antiporter |
|
|
Furosemide/Bumetanide
|
Loop- diuretics that enhance urine outflow.
Have to initially be secreted into the tubular lumen for them to initially work. |
|
|
You can figure out GFR from urine output.
TRUE or FALSE |
FALSE
|
|
|
Which substances will have a clearance of 0?
|
Glucose
Bicarbonate (most circumstances) |
|
|
Interstitial fluid osmolality (Cortex)
|
Consistently isotonic (300 mOsm/kg)
|
|
|
Interstitial fluid osmolality (Medulla)
|
Progressively increases from cortico-medullary border to tip of the papilla
human kidney gradient: 300 --> 1400 mOsm/kg; rat gradient 300 --> 3000 mOsm/kg |
|
|
Medulla Hypertonicity
|
Hypertonicity generated by deposition of high concentrations of NaCl and urea (particularly in the inner medulla)
|
|
|
The interstitial hypertonicity in the medulla is essential for urine concentration.
TRUE or FALSE |
TRUE
|
|
|
Maximal medullary osmolality
|
Maximal medullary osmolality species-dependent, and directly correlates with the length of the medulla-papilla and the percentage of long-looped nephrons (rabbit, 600-800 mOsm/kg; rat, 3000 mOsm/kg; mouse, 4000 mOsm/kg)
|
|
|
Thin Descending Limb
|
Reabsorbs H2O
Does NOT reabsorb NaCl |
|
|
Net Effect in Thin Descending Limb
|
Tubular fluid osmolality increases as it flows toward the papilla due to water reabsorption (300 --> 1200 mOsm in longest-looped nephrons)
TDL system capable of reabsorbing about 30-40 L H2O/day |
|
|
Ascending Limb
|
Reabsorbs NaCl
Does NOT reabsorb H2O |
|
|
Net Effect in Ascending Limb
|
20-25% of the filtered load of NaCl reabsorbed by this segment
AL generates hypotonic tubular fluid (the “diluting segment” of the nephron) |
|
|
Transport Mechanism in the Thick Ascending Limb
|
Dependent on Na+-K+-ATPase located on the basolateral membrane
Transport into the cell across the luminal membrane via Na+-K+-2Cl- co-transporter Reflux of entering K+ into tubular lumen via a K+-selective channel: i. generates lumen-positive potential which provides the driving force for paracellular transport of multiple cations (Na+, K+, Ca2+, Mg2+) ii. ensures adequate supply of K+ for Na+-K+-2Cl- cotransporter Na+-K+-2Cl- cotransporter inhibited by “loop diuretics” (e.g. furosemide (lasix); bumetanide) |
|
|
Limb “GT balance”
Potential Modulators of NaCl Reabsorption in the (TAL) |
↑ delivery (of NaCl) to TAL → ↑ reabsorption
|
|
|
Adrenal insufficiency
Potential Modulators of NaCl Reabsorption in the (TAL) |
↓ urine diluting and concentrating ability
|
|
|
Transport increased by?
Potential Modulators of NaCl Reabsorption in the (TAL) |
antidiuretic hormone
insulin glucagon isoproterenol |
|
|
Transport inhibited by?
Potential Modulators of NaCl Reabsorption in the (TAL) |
adenosine
dopamine bradykinin atrial natriuretic peptide |
|
|
”Passive” Reabsorption of NaCl in the Thin Ascending Limb
|
Na+-K+-ATPase-dependent “active” transport of NaCl very limited in thin limb
Interstitial urea creates a favorable concentration gradient for passive NaCl reabsorption |
|
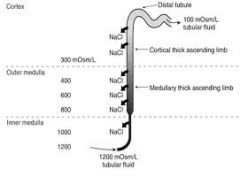
Tip of Loop
|
Interstitial fluid and tubular fluid osmolalities are both 1200 mOsm/kg, but
Interstitial (INT) osmolality = 50% urea / 50% NaCl = 600 mM urea / 300 mM NaCl (600 mOsm) Tubular fluid (TF) osmolality = 80% NaCl = 480 mM (960 mOsm) Thus: [NaCl]TF > [NaCl]INT meaning NaCl can be reabsorbed passively along its [ ] gradient |
|
|
Distal nephron
|
Distal convoluted tubule + connecting tubule
(cortical, medullary, inner medullary (papillary)) |
|
|
Distal delivery
|
10% filtered load of H2O
<10% filtered load of NaCl and KCl |
|
|
Distal nephron transport of H2O regulated by Antidiuretic hormone (ADH)
|
↑ ADH --> ↑ H2O reabsorption -->
↓ excretion) |
|
|
Distal nephron transport of NaCl regulated by Aldosterone
|
↑ Aldo --> ↑ NaCl reabsorption --> ↓ excretion
|
|
|
ADH present in collecting tubule
|
In the presence of ADH, collecting tubule is permeable to water
Favorable osmotic gradient (tubular fluid → interstitium) = water reabsorption (dashed arrows) thereby generating a concentrated (hypertonic) urine: ANTIDIURESIS |
|
|
ADH absent in collecting tubule
|
In the absence of ADH, collecting tubule essentially impermeable to water
Reabsorption of NaCl in the distal nephron (solid arrows) continues to dilute tubular fluid resulting in excretion of a hypotonic urine: DIURESIS |
|
|
The Intracellular Mechanism of ADH Action
|
Reabsorption of water by the collecting tubule system dependent on the presence of water channels (“aquaporins”) in the luminal membrane
ADH triggers the insertion of pre-existing AQP-2 channels into the membrane: ↑ ADH → ↑ cyclic AMP → ↑ translocation of channel-containing vesicles to luminal membrane → ↑ fusion and insertion (similar to exocytosis) → ↑ water permeability Process is reversible: ↓ ADH → vesicle retrieval → ↓ water permeability Note that AQP-3 channels are constitutively expressed in the basolateral membrane; permanently water permeable |
|
|
ADH triggers the insertion of pre-existing AQP-2 channels into the membrane
|
↑ ADH → ↑ cyclic AMP → ↑ translocation of channel-containing vesicles to luminal membrane → ↑ fusion and insertion (similar to exocytosis) → ↑ water permeability
Process is reversible: ↓ ADH → vesicle retrieval → ↓ water permeability |
|
|
Aquaporins
|
At least 3 aquaporin isoforms differentially distributed along the nephron
Ascending limb – low osmotic water permeability due to lack of luminal membrane aquaporins |
|
|
Countercurrent Multiplication (CM)
|
CM: An energy efficient mechanism for selective deposition of NaCl in the renal medulla
Maximal sustainable NaCl concentration difference across the thick ascending limb ~ 100 mM (transport of NaCl (lumen → interstitium) = passive backflux (interstitium → lumen)) If then only isotonic fluid (containing perhaps 100 mM NaCl) was delivered to the ascending limb, it would be impossible to maintain interstitial NaCl concentrations above 200 mM This problem circumvented by delivering pre-concentrated tubular fluid to the ascending limb |
|
|
A conceptual 3-step model
|
Reabsorption of NaCl by the ascending limb (ASC); retention in the medullary interstitium (INT)
Reabsorption of H2O by the descending limb; removal by vasa recta Isotonic fluid from proximal tubule into loop; hypertonic fluid into the ASC Repeat |
|
|
Deposition of Urea:
Urea Recycling |
Cortical and outer medullary collecting tubule cannot reabsorb urea therefore, in the presence of ADH tubular fluid urea [ ] will increase (↑ ADH → ↑ water reabsorption → ↑ tubular fluid urea [ ])
As fluid flows into the inner medullary collecting tubule, urea [ ] sufficiently high to promote passive reabsorption into the interstitium (ADH also increases permeability of this segment to urea) Some of the urea in the medulla will diffuse back into the tubular fluid in the thin descending and ascending limbs In the presence of ADH, this urea will be re-concentrated and reabsorbed: urea recycling But ↓ ADH → ↓ urea [ ] → ↓ reabsorption → ↑ urea excretion → ↓ medullary osmolality (Compare medulla osmolality in antidiuresis and diuresis on p. 48) ADH ultimately regulates medullary content of urea and NaCl for example: ↓ ADH → ↓ urea reabsorption → ↓ medullary urea → ↓ medullary osmolality (see above) and ↓ medullary osmolality → ↓ water reabsorption in thin descending → ↓ NaCl reabsorption in ascending limb → ↓ medullary osmolality If ADH levels increase, urea and NaCl will be re-deposited and ↑ medullary osmolality |
|
|
Maintenance of Hypertonic Medullary Interstitium:
Countercurrent Exchange |
Medullary blood supply essential for: i. nutrient supply
ii. controlled removal of reabsorbed H2O and NaCl Simply following concentration gradients, solute (urea/NaCl) diffuses into the capillary as blood flows into the medulla, but diffuses back out to the interstitium as it flows back out of the medulla Net effect: Countercurrent exchange in the vasa recta capillaries preserves the hypertonic gradient in the medullary interstitium Note that blood flow rate and solute content of plasma exiting medulla > than entering Both are indicative of the equally important function of vasa recta to remove all water reabsorbed by descending limbs and medullary collecting tubules, and most of the NaCl reabsorbed by the ascending limb |
|
|
High conc of urea can denature proteins. How do the cells survive such a difficult environment?
|
Cells can synthesize protective solutes (osmolytes) = prevent big swing in volume in medulla, and protect the cells against the high conc of urea
|
|
|
What output can be precisely controlled?
|
Urine output
|
|
|
Maximum Diuresis
Urine Volume Urine Osmolality |
Urine Volume: 20-25 L/day
Urine Osmolality: 50-75 mOsm/kg |
|
|
Maximum Antidiuresis
Urine Volume Urine Osmolality |
Urine Volume: 0.5 L/day
Urine Osmolality: 1200-1400 mOsm/kg |
|
|
ADH
|
arginine vasopressin (AVP): 1100 Da octapeptide
|
|
|
ADH Synthesis/Release
|
Synthesis: supraoptic and paraventricular nucleii of hypothalamus;
Release: posterior pituitary |
|
|
Deposition of Sodium Chloride:
Countercurrent Multiplication (CM) |
CM: An energy efficient mechanism for selective deposition of NaCl in the renal medulla
Maximal sustainable NaCl concentration difference across the thick ascending limb ~ 100 mM (transport of NaCl (lumen → interstitium) = passive backflux (interstitium → lumen)) If then only isotonic fluid (containing perhaps 100 mM NaCl) was delivered to the ascending limb, it would be impossible to maintain interstitial NaCl concentrations above 200 mM This problem circumvented by delivering pre-concentrated tubular fluid to the ascending limb |
|
|
A conceptual 3-step model to Countercurrent multiplication
|
Reabsorption of NaCl by the ascending limb (ASC); retention in the medullary interstitium (INT)
Reabsorption of H2O by the descending limb; removal by vasa recta Isotonic fluid from proximal tubule into loop; hypertonic fluid into the ASC Repeat |
|
|
Deposition of Urea:
Urea Recycling |
Cortical and outer medullary collecting tubule cannot reabsorb urea therefore, in the presence of ADH tubular fluid urea [ ] will increase (↑ ADH → ↑ water reabsorption → ↑ tubular fluid urea [ ])
As fluid flows into the inner medullary collecting tubule, urea [ ] sufficiently high to promote passive reabsorption into the interstitium (ADH also increases permeability of this segment to urea) Some of the urea in the medulla will diffuse back into the tubular fluid in the thin descending and ascending limbs In the presence of ADH, this urea will be re-concentrated and reabsorbed: urea recycling But ↓ ADH → ↓ urea [ ] → ↓ reabsorption → ↑ urea excretion → ↓ medullary osmolality ADH ultimately regulates medullary content of urea and NaCl for example: ↓ ADH → ↓ urea reabsorption → ↓ medullary urea → ↓ medullary osmolality (see above) and ↓ medullary osmolality → ↓ water reabsorption in thin descending → ↓ NaCl reabsorption in ascending limb → ↓ medullary osmolality If ADH levels increase, urea and NaCl will be re-deposited and ↑ medullary osmolality |
|
|
Maintenance of Hypertonic Medullary Interstitium:
Countercurrent Exchange |
Medullary blood supply essential for: i. nutrient supply
ii. controlled removal of reabsorbed H2O and NaCl Simply following concentration gradients, solute (urea/NaCl) diffuses into the capillary as blood flows into the medulla, but diffuses back out to the interstitium as it flows back out of the medulla Net effect: Countercurrent exchange in the vasa recta capillaries preserves the hypertonic gradient in the medullary interstitium Note that blood flow rate and solute content of plasma exiting medulla > than entering Both are indicative of the equally important function of vasa recta to remove all water reabsorbed by descending limbs and medullary collecting tubules, and most of the NaCl reabsorbed by the ascending limb |
|
|
High conc of urea can denature proteins. How do the cells survive such a difficult environment?
|
Cells can synthesize protective solutes (osmolytes) = prevent big swing in volume in medulla, and protect the cells against the high conc of urea
|
|
|
What output can be precisely controlled?
|
Urine output
|
|
|
Maximum Diuresis
Urine Volume Urine Osmolality |
Urine Volume: 20-25 L/day
Urine Osmolality: 50-75 mOsm/kg |
|
|
Maximum Antidiuresis
Urine Volume Urine Osmolality |
Urine Volume: 0.5 L/day
Urine Osmolality: 1200-1400 mOsm/kg |
|
|
ADH
|
arginine vasopressin (AVP): 1100 Da octapeptide
|
|
|
ADH Synthesis/Release
|
Synthesis: supraoptic and paraventricular nucleii of hypothalamus;
Release: posterior pituitary |
|
|
ADH secretion regulated by
|
hypothalamic osmoreceptors
volume receptors |
|
|
Sensitivity of the osmoreceptor system higher than baroreceptor system
TRUE or FALSE |
TRUE
(small changes in plasma osmolality can elicit large changes in ADH secretion) |
|
|
Volume status does influence the effects of osmolality on ADH secretion
|
e.g. ↓ volume → ↓ set point* and ↑ sensitivity to changes in osmolality
|
|
|
Set point
|
Plasma osmolality at which ADH secretion = 0
|
|
|
Relationship between Plasma ADH Concentration and Urine Concentrating Ability
|
Relatively small changes in ADH elicit large changes in urine osmolality
Recall that ↑ osmolality → ↓ urine volume |
|
|
The renal response to a decrease in total body water (↑ ADH; ↓ urine volume) will only minimize further losses of water.
TRUE or FALSE |
TRUE
|
|
|
hypothalamic osmoreceptors mechanism
|
↑ POSM → ↑ ADH → ↑ H2O reabsorption → ↓POSM (toward normal)
|
|
|
volume receptors mechanism
|
↓PVOL → ↑ ADH → ↑ H2O reabsorption → ↑ PVOL (toward normal)
|
|
|
The Thirst Mechanism
|
Maintenance of water balance requires control of water output and water intake
To restore normal water content quickly, water intake needs to be increased and: stimuli that regulate ADH release also regulate the thirst drive |
|
|
Syndrome of Inappropriate ADH Secretion (SIADH) Cause
|
Head trauma, encephalitis, meningitis
ADH secreting tumors (lung, pancreas) Drug-induced (nicotine, morphine, chemotherapeutic agents) |
|
|
Syndrome of Inappropriate ADH Secretion (SIADH) Effect
|
↑ ADH --> ↑ H2O reabsorption --> HYPONATREMIA
serum Na+ ↓ --> plasma osm ↓ influx of H2O (into brain cells) --> coma |
|
|
Diabetes Insipidus (D.I.) Cause
|
Hypothalamic (Central) D.I.
Nephrogenic D.I. |
|
|
Hypothalamic (Central) D.I.
|
↓ production or release of ADH
|
|
|
Nephrogenic D.I.
|
renal unresponsiveness to ADH
|
|
|
Nephrogenic D.I. Cause
|
Mutations in the ADH receptor:
↓ ADH binding --> ↓ cAMP --> ↓ H2O channel insertion Impaired synthesis/translocation of aquaporins Drugs (lithium, tetracyclines); hypokalemia |
|
|
D.I. Effect
|
↓ ADH --> ↓ H2O reabsorption --> HYPERNATREMIA
↑ serum Na+ --> ↑ plasma osm --> efflux of H2O (out of brain cells) --> coma |
|
|
Free water clearance
|
Provides a non-invasive assessment of diluting efficiency of the thick ascending limb
Free water clearance represents the amount of distilled water that must be added to (during antidiuresis) or removed from (during diuresis) urine to create an isotonic fluid When hypertonic urine is excreted a new term, free water reabsorption (TCH2O) is often substituted |
|
|
Free water clearance (CH2O) =
|
V – Cosm
|
|
|
Cosm = total osmolar clearance =
|
(V X Uosm) / Posm
Uosm and Posm = urine / plasma osmolality |
|
|
Free water clearance (CH2O) =
EXPANDED |
V - (V X UOSM) / POSM
|
|
|
Extracellular fluid (ECF) volume directly related to total body sodium (chloride) due to what (3)?
|
H2O shifts extracellular <--> intracellular
Effects on ADH secretion, thus collecting tubule H2O reabsorption Effects on thirst |
|
|
Maintenance of Extracellular Fluid Volume
|
Extracellular fluid (ECF) volume directly related to total body sodium (chloride)
Kidneys maintain constant ECF volume by adjusting NaCl excretion to match NaCl intake Typically 1% or less of the filtered load of sodium is excreted (<250 mEq/day) Na+ excretion primarily regulated by action of aldosterone on the cortical collecting tubule Aldosterone controls the fate of ~ 5-7% of the filtered load of NaCl |
|
|
Aldosterone in the cortical collecting tubule
|
Reabsorbs sodium and secretes potassium
(Principal driving force for this function is basolateral membrane Na+-K+-ATPase) |
|
|
Aldosterone controls reabsorption of Na+ by (3)?
|
↑ number of luminal membrane Na+ channels
↑ Na+-K+-ATPase (de novo synthesis of pumps) ↑ Krebs cycle enzyme synthesis (thus more ATP) --> ↑ ATPase activity |
|
|
Total body Na+ changes sensed as changes in what?
|
Effective Circulating Volume (ECV)
|
|
|
ECV Volume Receptors (BARORECEPTORS):
|
Cardiac Atria
Pulmonary Vasculature Carotid Sinus Aortic Arch Juxtaglomerular Apparatus |
|
|
Rate-limiting step in aldosterone release from adrenal cortex
|
Renin release by kidney
|
|
|
The renin-angiotensin-aldosterone system can be pharmacologically controlled at several points (4)
|
Renin inhibitors (aliskiren (Tekturna))
ACE (angiotensin converting enzyme) inhibitors (captopril) Angiotensin II receptor antagonists (saralasin; losartan) Aldosterone receptor antagonists (spironolactone) |
|
|
Renin secreted by?
|
Granular cells of the afferent arteriole
|
|
|
Renin regulated by three mechanisms
|
Intrarenal Baroreceptor
Sympathetic Nerves Macula Densa |
|
|
(Renin) Intrarenal Baroreceptor
|
Granular cells monitor pressure in afferent arteriole
↓ b.p. --> ↓ pressure in a.a --> ↑ renin |
|
|
(Renin) Sympathetic Nerves
|
Granular cells directly innervated
↓ b.p. --> ↑ r.s.n. activity --> ↑ renin |
|
|
(Renin) Macula Densa
|
Monitors changes in composition/flow rate of tubular fluid
↓ b.p. --> ↓ PGC --> ↓ GFR --> ↓ fluid flow rate to macula densa --> ↑ renin |
|
|
In response to decreased blood pressure (angiotensin)
|
Rapid-onset effects of AII: help restore “normal” b.p. despite low ECV (↑ TPR, ↑ CO)
Longer-onset effects of AII: restore normal ECV |
|
|
Longer-onset effects of AII (2)
|
↓ NaCl and H2O excretion
↑ NaCl and H2O intake |
|
|
Na+ Excretion with Increased ECV
|
Renal adjustments for changes in H2O intake are rapid (minutes) but renal compensation for a sudden alteration in NaCl intake can take several days
Due in part to the different mechanism(s) of ADH versus aldosterone action During transition, NaCl excretion does not match intake In “positive” (intake > excretion) or “negative” (intake < excretion) NaCl balance Increased ECV → ↑ ATRIAL NATRIURETIC PEPTIDE (ANP) from atrial myocytes ↑ ANP → ↑ NaCl and H2O excretion |
|
|
↑ ANP → ↑ NaCl and H2O excretion due to
|
Vasodilation of afferent arteriole → GFR
Inhibition of aldosterone secretion Inhibition of NaCl reabsorption in proximal tubule and collecting tubule Antagonized ADH action |
|
|
Inhibition of aldosterone secretion via (ANP)
|
Direct action on zona glomerulosa cells
Inhibition of renin release |
|
|
Antagonized ADH action via (ANP)
|
Inhibition of ADH release
Inhibition of ADH action on the collecting tubule |
|
|
Bartter’s Syndrome
|
Mutations in the gene expressing the Na+-K+-Cl- cotransporter (ENCC2) and/or luminal K+ (ROMK) channel in the thick ascending limb
|
|
|
Physiologic impact of Bartter’s Syndrome
|
Renal salt wasting (↓ NaCl reabsorption → ↑ NaCl excretion)
Volume depletion (↑ NaCl excretion → ↑ H2O excretion) Hyperreninemic hyperaldosteronism Hypercalciuria (↓ lumen positive potential) Hypokalemic metabolic alkalosis |
|
|
Edema (definition)
|
The accumulation of excess fluid in the interstitial (IS) space (typically > 2-3 L)
|
|
|
Edema Primary cause
|
Altered balance of Starling forces across systemic capillaries
e.g. ↓ plasma protein → ↑ IS volume → ↓ ECV → ↑ plasma protein but ↓ ECV → ↑ aldosterone → retention of NaCl and H2O → ↓ plasma protein → ↑↑ IS volume In this case the ↓ ECV does not reflect a ↓ in total ECF volume (and therefore NaCl); it is just redistributed |
|
|
Diuretics (definition)
|
Inhibit specific enzymes, transport proteins, hormone receptors or ion channels that are involved in transepithelial Na+ reabsorption
|
|
|
Diuretics net effect
|
Increased NaCl (and depending on the diuretic) water excretion
|
|
|
Proximal tubule carbonic anhydrase inhibitors
(Diuretic - enzyme) |
(e.g. acetazolamide)
Inhibit Na+HCO3- “reabsorption” |
|
|
Thick ascending limb “loop diuretics”
(Diuretic - transport protein) |
(e.g. furosemide, bumetanide)
Inhibit luminal Na+-K+-2Cl- cotransport |
|
|
Distal tubule luminal Na+-Cl- cotransport inhibitors
(Diuretic - transport protein) |
(e.g. thiazides)
Distal tubule luminal Na+-Cl- cotransport inhibitors |
|
|
Collecting tubule “K+-sparing” diuretics
(Diuretic - ion channel) |
(e.g.amiloride)
Inhibit luminal Na+ channels |
|
|
Collecting tubule “K+-sparing” diuretics
(Diuretic - hormone receptor) |
(e.g.spironolactone)
Aldosterone receptor antagonist |
|
|
98% of total body K+ is ?
|
Intracellular
(due to the role of the Na+-K+-ATPase) |
|
|
Normal plasma [K+] =
hyperkalemia [K+] = hypokalemia [K+] = |
Normal plasma [K+] = 4 mEq/L
hyperkalemia [K+] > 5.5 mEq/L hypokalemia [K+] < 3.5 mEq/L |
|
|
Total extracellular fluid K+ content =
|
~ 56 mEq (14 L X 4 mEq/L) therefore
Ingestion of relatively small amounts of K+ from the GI tract could have significant effects on plasma K+ concentration if retained within the ECF |
|
|
Dietary intake-induced changes in plasma [K+] prevented by (2)?
|
Rapid cellular uptake of K+ (epinephrine, insulin, aldosterone -->
↑ Na+-K+-ATPase) Slower renal excretion |
|
|
“Obligatory” reabsorption of 90% of the filtered load of K+ in?
|
Proximal tubule
Thick ascending limb |
|
|
Renal Tubular Transport of K+
|
Only 10 % of the filtered K+ load (FL) delivered to the distal nephron, BUT higher FL percentages excreted in the urine because K+ is SECRETED into the late distal and collecting tubule
Physiologic regulation of renal K+ excretion primarily achieved by controlling this rate of K+ secretion |
|
|
Typically, K+ is secreted into the distal and collecting tubule by?
|
Uptake across basolateral membrane via Na+-K+-ATPase
Efflux across luminal membrane Na+ reabsorption creates lumen-negative potential which also promotes K+ secretion |
|
|
K+ secretion in the distal and collecting tubule will have efflux across luminal membrane due to (2)?
|
K+ channels
K+-Cl- cotransporters |
|
|
In K+ depletion, K+ secretion ceases; low capacity K+ reabsorption will continue to reduce K+ excretion in the urine below the 10% FL delivered to the late distal/collecting tubule
Mechanistically reabsorption occurs by |
Uptake across luminal membrane via energy-dependent K+-H+ anti-porter
Efflux across basolateral membrane via K+-selective channels |
|
|
Physiologic Challenges to Extracellular Fluid K+ Homeostasis (2)
|
Hypertonicity: hypertonic ECF causes cells to shrink, which increases intracellular K+ concentration and thus increases K+ efflux; result hyperkalemia
Cell lysis will release K+ to the ECF; result (local) hyperkalemia e.g. exercise muscle breakdown |
|
|
Changes in ECF H+ can cause parallel changes in ECF K+
|
Metabolic acidosis (↑ ECF H+) → ↑ plasma K+
Metabolic alkalosis (↓ ECF H+) → ↓ plasma K+ |
|
|
Metabolic acidosis due to inorganic acids (HCl, H2SO4) increases plasma K+ to a much greater extent than a similar acidosis produced by organic acids (lactic acid, keto acids)
TRUE or FALSE |
TRUE
|
|
|
Respiratory acid-base disorders have little or no effect on plasma K+
TRUE or FALSE |
TRUE
|
|
|
An increase in ECF K+ concentration will result in increased K+ secretion and thus increased urine K+ excretion by (2)?
|
Directly ↑ Na+-K+-ATPase activity on distal nephron cells
Directly ↑ aldosterone secretion |
|
|
Directly ↑ aldosterone secretion in tubular potassium secretion will have what effect?
|
↑ Na+-K+-ATPase activity
↑ luminal membrane K+ permeability |
|
|
Effect of Tubular Fluid Flow
|
↑ flow → ↑ K+ secretion
|
|
|
↑ flow → ↑ K+ secretion due to (2)?
|
↑ flow minimizes the rise in tubular fluid K+ concentration
↑ flow → ↑ Na+ reabsorption → ↑ Na+-K+-ATPase activity → ↑ intracellular K+ |
|
|
Extended use of loop diuretics increases K+ excretion and leads to hypokalemia due to?
|
↓ K+ reabsorption in the thick ascending limb
↑ distal K+ secretion |
|
|
↑ distal K+ in diuretics secretion due to (2) ?
|
↑ distal tubular fluid flow
↑ distal Na+ reabsorption --> ↑ Na+-K+-ATPase --> ↑ intracellular [K+] |
|
|
Integrated Response to Hypocalcemia
|
Water, Na+ and K+ balance is dependent almost exclusively on renal function
In contrast, maintenance of normal plasma Ca2+ is dependent on integrated renal, intestinal and bone responses mediated by parathyroid hormone (PTH) Consider the response to a reduction in plasma Ca2+ (hypocalcemia) Resorption of bone also releases HPO42- potentially causing hyerphosphatemia Prevented by inhibitory effect of PTH on renal HPO42- reabsorption |
|
|
Renal Tubular Effects of Parathyroid Hormone (PTH)
|
↑ Ca2+ reabsorption in the distal tubule
↓ HPO42- reabsorption in the proximal tubule |
|
|
↑ Ca2+ reabsorption in the distal tubule due to?
|
Stimulation of Ca2+ ATPase and Na+-Ca2+ exchanger on basolateral membrane
|
|
|
↓ HPO42- reabsorption in the proximal tubule due to?
|
Inhibition of Na+-HPO42- co-transporter on the luminal membrane
|
|
|
“Normal” pH range:
Survival limits: |
“Normal” pH range: 7.37 - 7.42 Survival limits: 6.80 - 8.00
|
|
|
H+ constantly produced by normal metabolism via two principal sources
|
“Volatile” Acid: ~15-20,000 mmol/day of CO2 generated by oxidative metabolism
(Normally, volatile acid not a problem since it is efficiently eliminated by the lungs) “Fixed” (non-volatile) Acid: ~ 50 mmol/day of inorganic and organic acid generated (for example) by amino acid metabolism (Fixed acid increases with for example, exercise (lactic acid); diabetes mellitus (ketoacid)) |
|
|
Three “lines of defense” help prevent fixed acid-induced acidification of body fluids
|
Physicochemical buffering
Respiratory compensation (CO2 elimination) Renal compensation (H+ excretion; generation of HCO3-) |

