![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
71 Cards in this Set
- Front
- Back
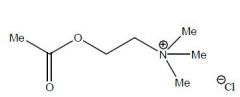
Name this structure and class
|
Acetylcholine Chloride
Muscarinic and Nicotinic Agonist |
|
|
Draw Acetylcholine Chloride
|
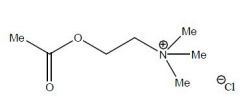
|
|
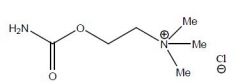
Name this drug and class
|
Carbachol Chloride
Non-selective Muscarinic/Nicotinic agonist |
|
|
Draw Carbachol Chloride
|
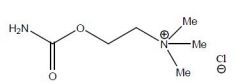
|
|
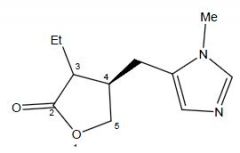
Name this compound and class
|
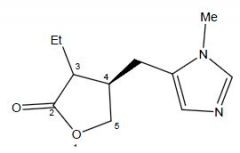
Pilocarpine
Muscarinic agonist |
|
|
Draw Pilocarpine
|
|
|

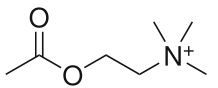
Name this drug
|
Acetylcholine
|
|
|
Draw Acetylcholine
|
|
|
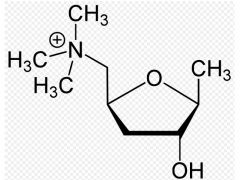
Name this drug
|
Muscarine
|
|
|
Draw Scopolamine
|
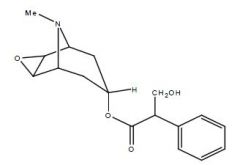
|
|

Name this drug
|
Telenzepine
|
|
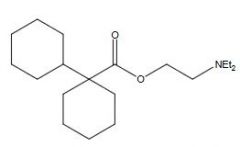
Name this drug
|
Dicyclomine
|
|
|
Draw Benztropine
|
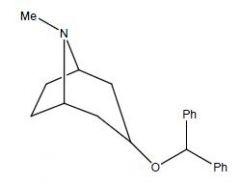
|
|
Name this drug
|
Methantheline
|
|
|
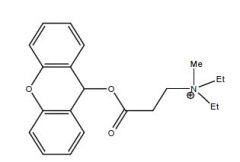
Draw Methantheline
|
|
|
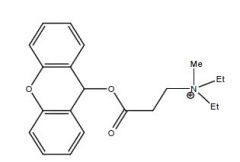
Name this drug
|
Propantheline
|
|
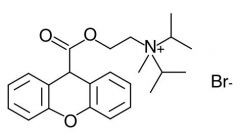
Name this drug
|
Succinylcholine
|
|
|
Draw Succinylcholine
|
|
|
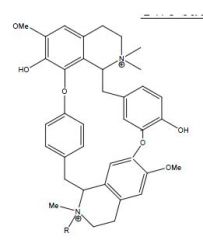
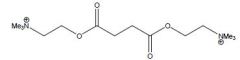
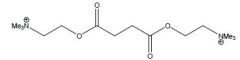
Name the two possible drugs for this structure
|
Turocurarine R=H
Metocurine R=Me |
|
|
Draw the structures for Tubocurarine and Metocurine
|
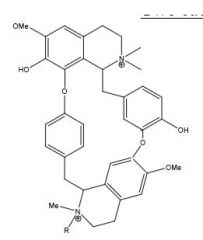
|
|
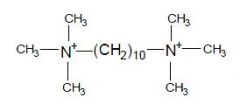
Name this drug
|
Decamethonium
|
|
|
Draw Decamethonium
|
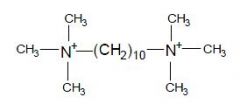
|
|
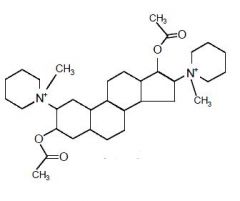
|
Draw Pancuronium
|
|
|
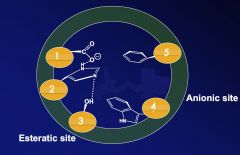
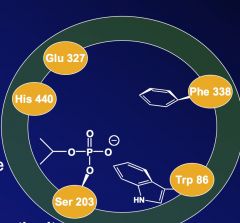
Name the amino acids in the active site of acetylcholinesterase.
|
1. Glu 327
2. His 440 3. Ser 203 4. Trp 86 5. Phe 338 |
|
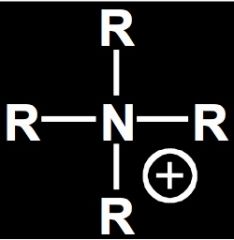
Name this molecule.
|
Tetraalkylammonium ion
|
|
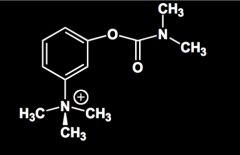
Name this molecule.
|
Neostigmine.
|
|
What does the image depict?
|
An aged enzyme after exposure to a nerve agent.
|
|
|
Draw isofluorophate/ DFP/Floropryl.
|
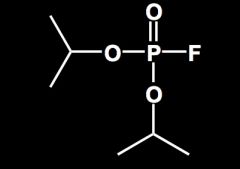
|
|
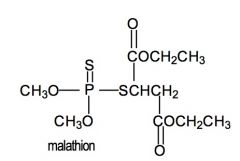
What is malathion used for?
|
Insecticide.
|
|
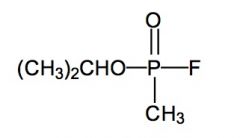
Name the above molecule.
|
Sarin (GB); nerve agent.
|
|
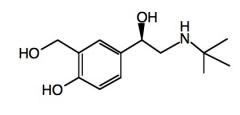
Name the above compound.
|
Albuterol
|
|

Name the above structure, and the parent compound it stems from.
|
Aziridinium ion; beta-haloalkylamines
|
|
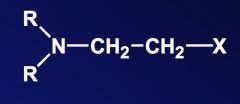
Name the above compound.
|
beta-haloalkylamine
|
|

Name the above compound.
|
Biolterol.
|
|
|
Draw a structure of colterol.
|
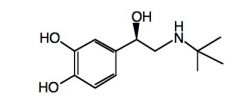
|
|
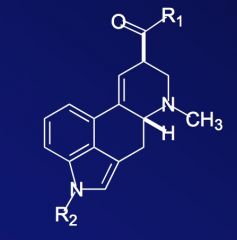
What group of compounds is the above scaffold representative of?
|
Ergot derivatives.
|
|

Name the above compound.
|
Dichlorisopreterenol
|
|
|
Draw a structure of isoproterenol.
|
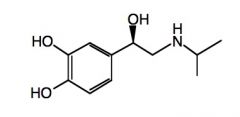
|
|
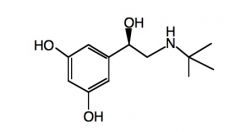
Name the above compound.
|
Metaproterenol
|
|
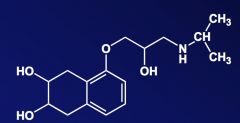
Name the above compound.
|
Nadalol
|
|
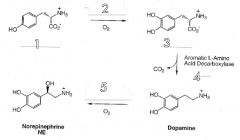
Fill in the missing enzymes/intermediates.
|
1. L-Tyrosine
2. Tyrosine Hydroxylase 3. L-DOPA 4. PLP 5. Dopamine beta-hydroxylase |
|
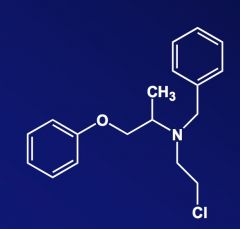
Name the above compound.
|
Phenoxybenzamine
|
|
|
Draw a structure of Regitine.
|
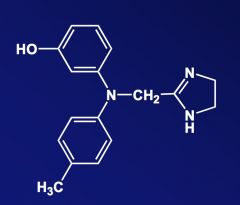
|
|
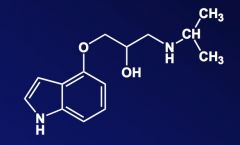
Name the above compound.
|
Pindolol
|
|
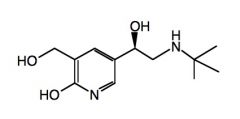
Name the above compound.
|
Pirbuterol
|
|
|
Draw a structure of Salmeterol.
|
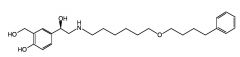
|
|
|
Draw a structure of Propanolol.
|
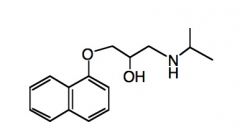
|
|
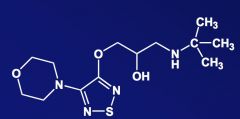
Name the above compound.
|
Timolol
|
|
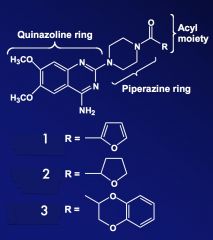
Name the compounds that correspond to the R groups.
|
1. Prazosin
2. Terazosin 3. Doxazosin |
|
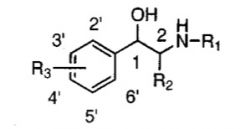
What receptor do R1 substituents influence the activity of?
|
beta; act as an agonist
|
|
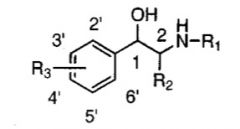
True or False: Larger derivatives at R1 favor beta 1 receptors over beta 2 receptors.
|
False.
|
|
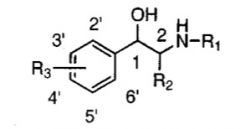
Alpha selectivity is observed in what type of substitution at R2?
|
Methyl
|
|
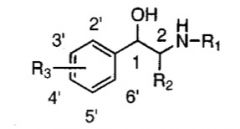
If a ring with a single hydroxy at 3' is substituted, which type of receptor specificity is observed?
|
alpha selectivity
|
|
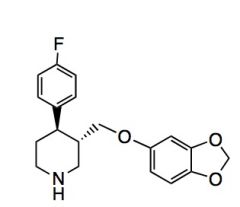
Name the above compound.
|
Paroxetine/Paxil
|
|
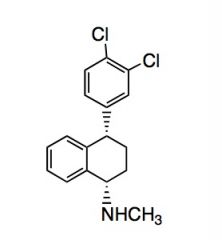
Name the above compound.
|
Sertraline/Zoloft
|
|

Name the above compound.
|
Citalopram/Celexa
|
|

Name the above compound.
|
Alosetron
|
|
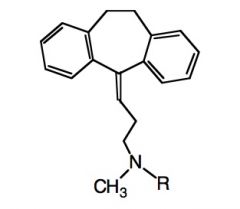
Name the above compound when (1) R=CH3 and (2) R= H
|
1) Amitryptilline
2) Nortyrptilline |
|
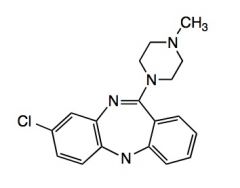
Name the above compound.
|
Clozapine
|
|
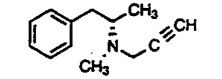
Name the class of drug the above compound belongs to.
|
MAO inhibitor (deprenyl; irreversible)
|
|
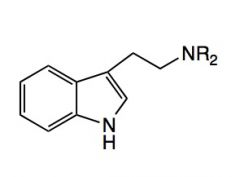
Name the above compound.
|
Dimethyltryptamine
|
|
|
Draw fluoxetine/Prosac
|
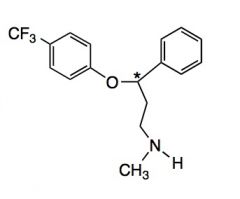
|
|
|
Draw Imipramine/Desipramine.
|
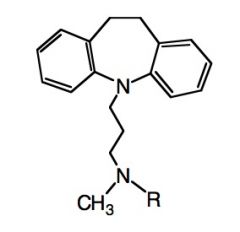
Where imipramine corresponds to R=CH3 and desipramine corresponds to R=H.
|
|
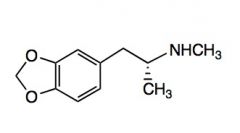
Name the above compound.
|
MDMA
|
|

Name the above compound.
|
Mescaline
|
|
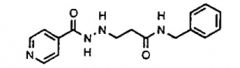
Name the class of drug the above compound belongs to.
|
MAO inhibitor (nialamide; irreversible)
|
|
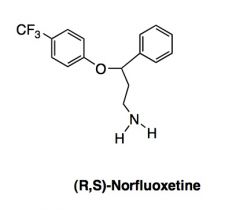
Where and how is the above compound produced?
|
Produced by metabolism of fluoxetine by CYP2D6 once ingested.
|
|

Name the above compound.
|
Ondansetron/Zofran
|
|
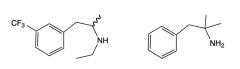
What popular drug combination is pictured?
|
Phen-Fen
|
|
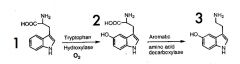
Name the structures above.
|
1. Tryptophan
2. 5-Hydroxyltryptophan (5-HTP) 3. Serotonin (5-HT) |
|
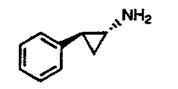
What class of drug does the above compound belong to?
|
MAO Inhibitor (tranylcypromine; irreversible)
|

