![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
172 Cards in this Set
- Front
- Back
|
Carbohydrates
|

aldehydes & ketones containing # hydroxy groups on unbranched C chain & chem derivatives
|
|
|
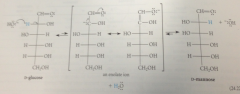
Most common sugars have mlclr formulas that fit
|
hydrate of carbon pattern - Cn(H2O)m
|
|
|
sucrose (table sugar)
|
C12(H2O)11 or C12H22O11
|
|
|
glucose & fructose
|
prevalent in honey, C6(H2O)6 or C6H12O6
|
|
|
Carbohydrates as abundant organic cmpds
|
in polymerized form as cellulose, account for 50-80% of dry weight of plants, major source of food sucrose (table sugar) & lactose (milk sugar) shells of arthropods such as lobsters
|
|

|
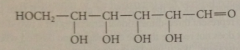
|
|

|
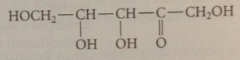
|
|
|
aldose
|
carb w aldehyde carbonyl group
|
|
|
ketose
|
carb w ketone carbonyl group
|
|
|
hexose
|
6C carb
|
|
|
pentose
|
5 C carb
|
|
|
pentulose
|
5C ketose
|
|
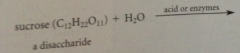
|
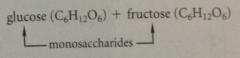
|
|
|
monosaccharides
|
cannot be converted into simpler carbs by hydrolysis i.e. glucose, fructose
|
|
|
disaccharide
|
sucrose - can be converted by hydrolysis into two monosaccharides
|
|
|
trisaccharides
|
can be hydrolyzed to 3 monosaccharides
|
|
|
oligosaccharides
|
to a few monosaccharides
|
|
|
polysaccharides
|
to a large number of monosaccharides
|
|
|
Are carbs soluble in water?
|
Yes, many hydroxy groups
|
|
|
Fischer projections
|
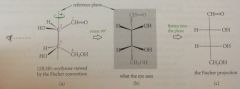
almost all carbs are chiral and most have 1+ asymm C - many carbs have several contiguous asymm C in unbranched chain
|
|
|
Fischer rules
|
projection based on eclipsed mlclr conformation, bonds connecting asymm C arranged in vertical line, asymm C located @ intersections of vertical & horizontal bonds (not drawn explicitly) vertical bonds to asymm C recede, horizontal bonds emerge toward observer
|
|
|
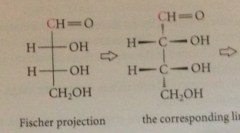
do fischer projections convey info about mlclr conformations?
|
no - only absolute configuration of each asymm C
|
|
|
|
|
|
can a fischer projection be turned 180?
|
Yes, in the plane of the paper
|
|
|
Can a Fischer projection be turned 90?
|
No
|
|
|
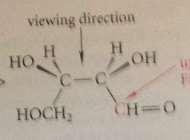
Can a Fischer projection be lifted from the plane of the paper and turned over?
|
No
|
|
|
Can the 3 groups at either end of a Fischer projection be interhanged in a cyclic permutation?
|
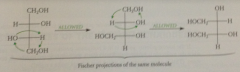
Yes, all three groups can be moved at the same time in a closed loop so that each occupies an adjacent position
|
|
|
An interchange of any 2 groups bound to an asymm C changes
|
the configuration of that C
|
|
|
it is easy to recognize enantiomers & meso cmpds from Fischer projections bc
|
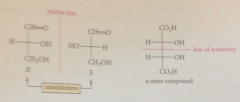
planes of symmetry in actual mlcs reduce to lines of symmetry in their projections
|
|
|
If the group of lowest priority is in either of the two vertical positions
|
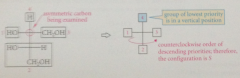
apply R,S priority rules to remaining three groups
|
|
|
If lowest priority group is in a horizontal position
|
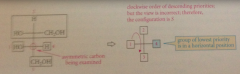
reverse the assignment
|
|
|
Each diastereomer is
|
a diff carb w diff properties known by a diff name
|
|
|
fam of aldoses
|
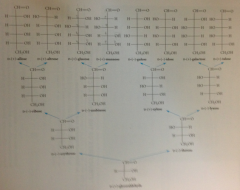
|
|
|
each monosaccharide has an
|
enantiomer
|
|
|
how to apply d,L system
|
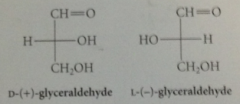
configuration of naturally occurring triose (+) glyceraldehyde is designated as D, enantiomer is L,
|
|
|
aldoses or ketoses written in Fischer projection w C in straight vertical line & C numbered consecutively as would be in systematic nomenclature so that
|
carbonyl C receives lowest possible #
|
|
|
Asymm C of ___ # is designated as a reference C
|
highest - if this C has the H, OH, CH2OH groups in same relative configuration as same three groups of D-glyceraldehyde, carb has D configuration
|
|
|
Is there a general correspondence btwn configuration & sign of optical rotation?
|
no
|
|
|
difference btwn R,S & D,L system
|
R,S used to specify configuration of each asymm C atom in a mlc D,L specifies particular enantiomer of a mlc that may contain many asymm C
|
|
|
important aldoses to know
|
D-Glucose, D-mannose, D-galactose
|
|
|
epimers
|
diastereomers that differ in configuration at only one of several asymm C
|
|
|
important aldopentose
|
D-Ribose
|
|
|
D-Fructose
|
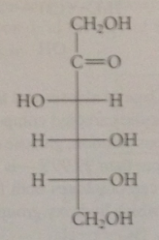
important naturally occurring ketose
|
|
|
y or delta-hydroxy aldehydes exist predominantly as
|
cyclic hemiacetals
|
|
|
|
|
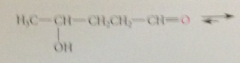
|
|
|
|
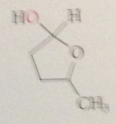
aldoses and ketoses exist primarily as
|
cyclic hemiacetals
|
|
|
in many carb both 5 & 6 membered cyclic hemiacetals are possible depending on
|
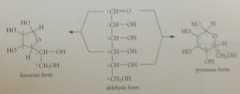
which hydroxy group undergoes cyclization
|
|
|
furanose
|
5 membered cyclic acetal form of a carb
|
|
|
pyranose
|
6-membered cyclic acetal form of a carb
|
|
|
aldohexoses & aldopentoses exist predominantly as
|
pyranoses in aq. soln, but furanose forms of some carbs are important
|
|
|
to name a cyclic hemiacetal form of a carb
|
start w prefix derived from name of carb followed by a suffix that dictates the type of hemiacetal ring
|
|
|
the furanose or pyranose form of a carb has
|
one or more asymm C than the open chain form - C1 in case of aldoses
|
|
|
how many possible diastereomers of D-glucopyranose?
|
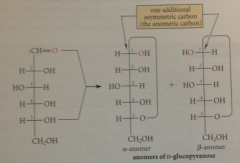
two
|
|
|
anomers
|
two cyclic forms of a carb differ in configuration only at their hemiacetal C aka cyclic forms of carbs that are epimeric at the hemiacetal C
|
|
|
anomeric carbon
|
hemiacetal carbon (C-1 of an aldose)
|
|
|
anomers are named with
|
greek letters a & b
|
|
|
in the a-anomer the hemiacetal OH group
|
is on the same side of the Fischer projection as the O at the configurational C
|
|
|
the b-anomer, the hemiacetal OH group
|
is on the side of the Fischer projection opposite the O at the configurational C
|
|
|
when a carb ring is drawn w the anomeric C on the right & ring O in the rear
|
substituents on left in Fischer projection are up in Haworth projection or chair structures, groups on right are down in Haworth projection or chair structures
|
|
|
Altho 5 membered rings of furanoses are nonplanar, they are close enough to planarity that
|
Haworth projections are good approximations to their actual structures
|
|
|
Haworth projection of B-D-ribofuranose
|
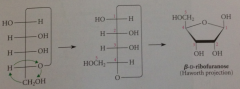
|
|
|
when pure a-D-glucopyranose is dissolved in water its specific rotation is found to be
|
+112 degrees mL g-1 dm-1
|
|
|
with time the specific rotation of the soln
|
dec, reaches stable +52.7 degrees
|
|
|
When pure B-D-glucopyranose is dissolved in water it has a specific rotation of
|
+18.7 degrees
|
|
|
mutarotation
|
change of optical rotation w time
|
|
|
Mutarotation occurs when
|
pure anomers of other carbs are dissolved in aq soln
|
|
|
Mutarotation of glucose is caused by
|
conversion of a- and b-glucopyranose anomers into an equil mix of both, formed from either pure a-D-glucopyranose or B-D-glucopyranose
|
|
|
Mutarotation is cat by
|
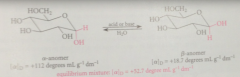
acid & base, but also occurs slowly in pure water
|
|
|
mutarotation is characteristic of
|
cyclic hemiacetal forms of glucose
|
|
|
aldehyde cannot undergo mutarotation bc
|
an aldehyde C is not an asymm C
|
|
|
Mutarotation was one of the phenomena that suggested that aldoses might exist as
|
cyclic hemiacetals
|
|
|
Mutarotation occurs by
|
opening of the pyranose ring to the free aldehyde form, the reverse of hemiacetal formation
|
|
|
180 rotation about the C-C bond to the carbonyl group permits
|
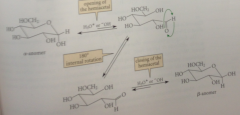
reclosure of the hemiacetal ring by the rxn of the hydroxy group @ the opposite face of the carbonyl C
|
|
|
mutarotation of glucose is due to
|
interconversion of 2 pyranose forms
|
|
|
other carbs undergo
|
more complex mutarotations
|
|
|
the structures of the cyclic hemiacetal forms of D-fructose can be derived from
|
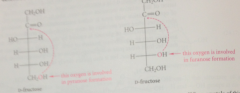
its carbonyl (ketone) form
|
|
|
the crystalline form of D-fructose
|
B-D-fructopyranose
|
|
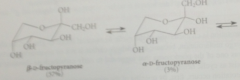
When crystals of B-D-fructopyranose are dissolved in water
|
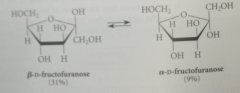
it equilibrates to both pyranose & furanose forms
|
|
|
Does glucose in soln contain furanose forms?
|
Yes but in very small amts ~ 0.2% each
|
|
|
A single hexose can exist in at least 5 forms
|
acyclic aldehyde or ketone form, a & b-pyranose forms, a & B- furanose forms
|
|
|
most aldohexoses & aldopentoses exist primarily as
|
pyranoses, altho few have substantial amts of furanose forms
|
|
|
most monosaccharides contain relatively
|
small amts of their noncyclic carbonyl forms
|
|
|
mixtures of a & B-anomers
|
are usually found, but exact amts of each vary from case to case
|
|
|
fraction of any form in soln @ equil
|
determined by its stability relative to that of all other forms
|
|
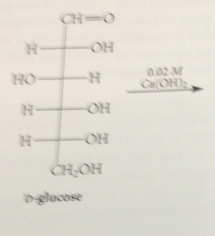
Lobry de Bruyn-Alberda van Ekenstein rxn - in base, aldoses & ketoses rapidly equilibrate to
|
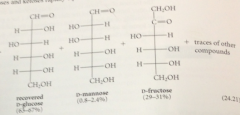
mixtures of other aldoses & ketoses
|
|
|
altho glucose in soln exists mostly in its cyclic hemiacetal forms, it is also in equilibrium w
|
a small amt of its acyclic aldehyde form
|
|
|
This aldehyde like other carbonyl cmpds w a-hydrogens
|
ionizes to give small amts of its enolate ion in base
|
|
|
Protonation of this enolate ion @ one face of the db gives back ___
|
glucose, protonation @ the other face gives mannose
|
|
|
enediol
|
enoalte ion protonated on oxygen to give a new enol
|
|
|
enediol contains a
|
hydroxy group @ each end of the db
|
|
|
enediol derived from glucose is simultaneously
|
the enol of not only the aldoses glucose & mannose but also the ketose fructose
|
|
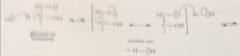
|
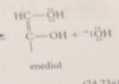
|
|
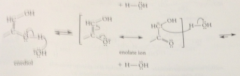
|
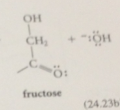
|
|
|
conversion of D-glucose-6-phosphate into D-fructose-6-phosphate occurs
|
in the breakdown of D-glucose (glycolysis), series of rxns by which D-glucose is utilized as a food source
|
|
|
Bc biochem rxns occur near pH 7
|
too little hydroxide ion is present to cat rxn
|
|
|
Instead rxn cat by an enzyme
|

D-glucose-6-phosphate isomerase & involves enediol intermediate
|
|
|
conversion of D-glucose derived from corn into D-fructose is
|
enzyme-cat process, involves similar rxns - central to commercial prod of high-fructose corn syrup, widely used sweetener
|
|
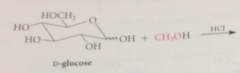
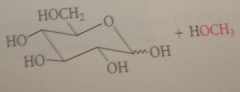
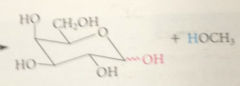
most monosaccharides react w alcohols under acidic conditions to yield
|
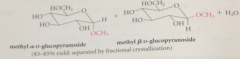
cyclic acetals
|
|
|
glycosides
|
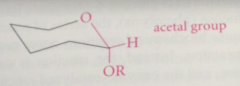
special types of acetals in which one of the O of the acetal group is the ring O of the pyranose or furanose
|
|
|
contrast rxn of a cyclic hemiacetal (i.e. glucopyranose) w corresponding rxn of an ordinary aldehyde under same conditions
|
glycoside one -OR group is incorporated, formation of aldehyde acetal 2 -OR groups are incorporated
|
|
|
glycosides are named
|
as derivatives of the parent carb
|
|
|
pyranoside
|
indicates that the glycoside ring is 6-membered
|
|
|
furanoside
|
5-membered ring
|
|
|
Glycoside formation
|
like acetal formation, is cat by acid, involves a-alkoxy carbocation intermediate
|
|

|
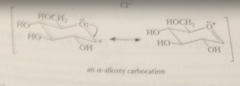
|
|
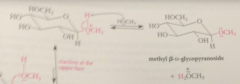
|
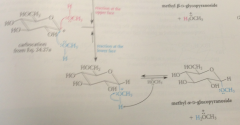
|
|
|
like other acetals, glycosides are
|
stable to base, but in dilute aq acid, hydrolyzed back to parent carb
|
|
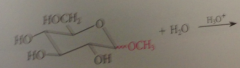
|
|
|
|
many cmpds exist naturally as
|
glycosides
|
|
|
glycoside formation plays an important role in
|
removal of some chem from the body - carb is joined to an OH group of substance to be removed, added carb group makes substance more soluble in h2o, hence more easily excreted
|
|
|
like simple methyl glycosides, glycoside of a natural prod can be
|
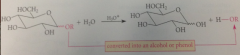
hydrolyzed to its component alcohol or phenol & carb
|
|
|
disaccharides
|
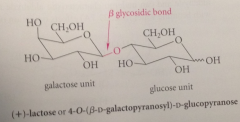
2 monosaccharides connected by a glycosidic linkage
|
|
|
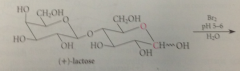
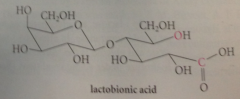
(+)-Lactose
|
example of a disaccharide, present in about 4.5% cows milk, 6-7% human milk.. D-glucopyranose mlc linked by O @ C-4 to C-1 of D-galactopyranose, so effectively glycoside in which galactose is the carb & glucose is the alcohol
|
|
|
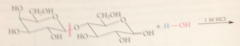
glycosidic linkage is an acetal & acetals
|
hydrolyze under acidic conditions
|
|
|
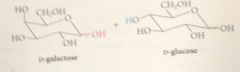
|
|
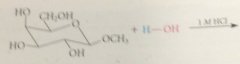
|
|
|
|
disaccharide structural basis
|
carb that can be hydrolyzed to 2 monosaccharides
|
|
|
Hydrolysis occurs at the
|
glycosidic bond btwn the 2 monosaccharide residues
|
|
|
stereochem of glycosidic bond in (+) lactose
|
B
|
|
|
B stereochem
|
stereochem of O linking 2 monosaccharide residues in the glycosidic bond corresponds to B-anomer of D-galactopyranose
|
|
|
higher animals possess an enzyme, B-galactosidase
|
cat hydrolysis of B-glycosidic linkage near neutral pH
|
|
|
hydrolysis allows lactose to act as
|
source of glucose, a-glycosides of galactose inert to action of this enzyme
|
|
|
bc C-1 of the galactose residue in (+)-lactose is involved in a glycosidic linkage
|
it cannot be oxidized
|
|
|
C-1 of the glucose residue is part of
|
a hemiacetal group which is in equil w free aldehyde & can undergo characteristic aldehyde rxns
|
|
|
|
|
|
reducing sugars
|
carbs such as (+)-lactose that can be oxidized bc they reduce oxidizing agents
|
|
|
glucose residue said to be
|
at the reducing end of the disaccharide
|
|
|
galactose residue is at the
|
nonreducing end
|
|
|
bc of its hemiacetal group (+) lactose
|
also undergoes many other rxns of aldose hemiacetals such as mutarotation
|
|
|
(+) sucrose
|
table sugar, another important disaccharide
|
|
|
sucrose consists of
|
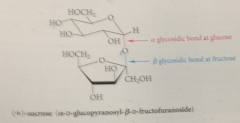
D-glucopyranose residue & a D-fructofuranose residue connected by glycosidic bonds @ anomeric C of both monosaccharides
|
|
|
glycosidic bond in (+) sucrose is diff from one in lactose
|
only one of the residues of lactose (galactose residue) contains an acetal (glycosidic) C, both residues of +sucrose have an acegtal C
|
|
|
glycosidic bond in + sucrose bridges
|
C2 of fructofuranose residue & C1 of glycopyranose residue
|
|
|
carbonyl C become
|
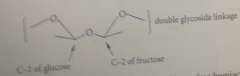
the acetal or hemiacetal C in the cyclic forms
|
|
|
neither the fructose nor the glucose part of sucrose
|
has a free hemiacetal group, so +sucrose cannot be oxidized by bromine water nor undergo mutarotation
|
|
|
nonreducing sugars
|
carbs such as +sucrose that cannot be oxidized by bromine water
|
|
|
sucrose is hydrolyzed by
|
aq. acid or by enzymes (invertases) to an equilmolar mixture of D-glucose & D-fructose, sometimes called invert sugar
|
|
|
as hydrolysis of sucrose proceeds
|
positive rotation of soln changes to a neg rotation characteristic of the glucose-fructose mixture
|
|
|
Rotation is negative because the strongly negative rotation
|
of fructose has a greater magnitude than the positive rotation of glucose (dextrose)
|
|
|
Fructose
|
sweetest of the common sugars, accounts for intense sweetness of honey
|
|
|
polysaccharides
|
any # of monosaccharide residues can be linked together w glycosidic bonds to form chains
|
|
|
cellulose
|
principal structural component of plants, most abundant organic cmpd on earth
|
|
|
cotton
|
pure cellulose
|
|
|
wood
|
cellulose combined w polymer called lignin
|
|
|
5 x 10^14 kg of cellulose is
|
biosynthesized & degraded annually on the earth
|
|
|
cellulose is a regular polymer
|
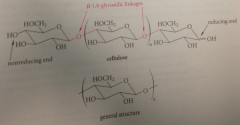
of D-glucopyranose units connected by B-1,4-glycosidic linkages
|
|
|
polysaccharides can be hydrolyzed?
|
yes
|
|
|
mammals lack the enzymes that cat hydrolysis of B-glycosidic linkages of cellulose
|
why humans cannot digest grasses but cattle can (bacteria in their rumens provide appropriate enzymes that break down plant cellulose to glucose)
|
|
|
uses of processed celulose
|
spun into fibers (rayon) or made into wraps (cellophane)
|
|
|
nitration of cellulose hydroxy groups
|
gives nitrocellulose, a powerful explosive
|
|
|
cellulose acetate
|
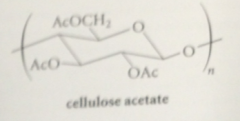
hydroxy groups of cellulose are esterified w acetic acid, known by trade names Celanese, Arnel, so on, used in knitting yarn & decorative household articles
|
|
|
cellullose as an alternative E source
|
biomass is largely cellulose & cellulose is polymerized glucose
|
|
|
glucose derived from hydrolysis of cellulose
|
can be fermented to ethanol, which can be used as a fuel (as in gasohol) & plants obtain E to manufacture cellulose from sun
|
|
|
important research: how to convert abundant sources of cellulose such as wild grasses
|
into glucose w/o expending large amt of E - soln would reduce or elim need to use cultivated crops such as corn, a source of ethanol
|
|
|
starch
|
like cellulose, a polymer of glucose, mixture of 2 diff types of glucose polymer
|
|
|
amylose
|
glucose units are connected by a-1,4-glycosidic linkages
|
|
|
conceptually chem diff btwn amylose & cellulose
|
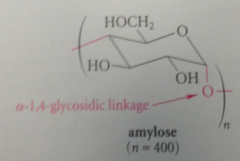
stereochem of glycosidic bond
|
|
|
amylopectin
|
branched polysaccharide, contains relatively short chains of glucose units in a-1,4-linkages & branches that involve a-1,6-glycosidic linkages
|
|
|
starch
|
important storage polysaccharide in corn, potatoes & other starchy veggies
|
|
|
Humans have enzymes that cat hydrolysis of
|
a-glycosidic bonds in starch, can use starch as source of glucose
|
|
|
Chitin
|
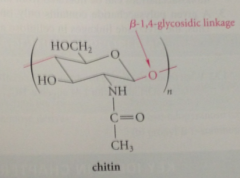
polysaccharide that occurs widely in nature - notably in shells of arthropods (lobsters, crabs)
|
|
|
Chitin is a polymer of
|
N-acetyl-D-glucosamine
|
|
|
Residues of chitin are connected by
|
B-1,4-glycosidic linkages within chitin polymer
|
|
|
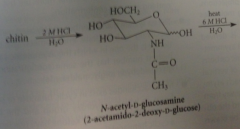
N-Acetyl-D-glucosamine is liberated when
|
chitin is hydrolyzed in aq. acid
|
|
|
Stronger acid brings abotu hydrolysis
|
of the amide bond to give D-glucosamine hydrochloride & acetic acid
|
|
|
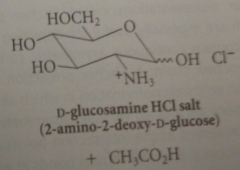
|
|
|
amino sugars
|
glucosamine & N-acetylglucosamine - a number occur in nature
|
|
|
Amino sugars linked to proteins (glycoproteins)
|
are found at the outer surfaces of cell membranes, some responsible for blood-group specificity
|
|
|
polysaccharides
|
mostly long chains w some branches - no highly cross-linked, 3D networks - some cyclic oligosaccharides known
|
|
|
linkages btwn monosaccharide units
|
in every case glycosidic linkages so monosaccharides can be liberated from all polysaccharides by acid hydrolysis
|
|
|
A given polysaccharide contains
|
only one stereochem type of glycoside linkage, so glycoside linkages in cellulose are all B, those in starch are all alpha
|

