![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
159 Cards in this Set
- Front
- Back
|
When ________ are more elongated & myosin & actin are LESS overlaped, a greater force of contraction can be generated. (Frank-Starling Mechanism) HOWEVER, if it is so elongated that there is NO OVERLAP, cardiac myocytes contractility is greatly reduced. |
Sarcomeres |
|
|
How does the Frank-Starling Mechanism respond in CHF? |
1. heart starts to fail (blood not effectively pumped out of ventricles)--> ventricles begin to dilate 2. Sarcomeres elongate as ventricles dilate--> improves contractility of myocardium & maintains CO 3. As ventricles dilate further sarcomeres become too elongated (no overlap)--> reduced contractility = "cardiac decompensation" fulminant CHF 4. death |
|
|
CHF is characterized by ______&_______ & usually due to systolic dysfunction, but eventually both systolic (dec contractility & EF) & diastolic (dec SV) dysfunction are involved |
dec cardiac output (forward failure) & damming back of blood into venous cirulation (backward failure)
|
|
|
What are the adaptive responses in CHF that attempt to maintain CO? |
1sr- Frank-Starling Mechanism (dilation--> inc contractility) 2nd- Activation of neurohormonal system |
|
|
What occurs in the Activation of the neurohormonal system? |
1. release of NE--> inc HR & conractility 2. activation of renin-angiotensin system--> inc blood volume ^both in CO then inc blood volume stimulates; 3. release of atrial natriuretic peptide--> dec blood volume ^attempt to prevent edema from too high blood volume |
|
|
Cardiac hypertrophy usually occurs prior to CHF. Describe. |
inc mechanical work (stenosis,hypertrophy,etc)--> inc protein synthesis--> inc monocyte (heart) size & mass |
|
|
The inc protein synthesis leads to induction of immediate-early genes, re-expression of fetal contractile proteins & an inc in DNA. What problems does this lead to? |
impaired myocyted fxn & inc metabolic requirements
(fetal contractile proteins are not as functional as adult myocytes) |
|
|
inc metabolic requirements & inc intercapillary distance causes ______________ due to ischemia (inadequate microvasculature) & apoptosis (misfolded proteins, damaged DNA) |
loss of myocytes |
|
|
The loss of myocytes initially causes systolic but also leads to impaired diastolic filling as _________ replaces myocytes |
fibrous tissue replaces myocytes |
|
|
Pt w hypertrophy (esp LV) due to hypertension, valvular disease, MI, etc will eventually have ______ &/or _______ |
heart failure (CHF) &/or arrythmias
(early CHF may be single sided hypertrophy) |
|
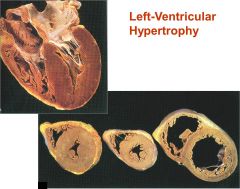
LVH w/o dilation (far L heart) is typical in pt w _____________
(middle is normal & R is LVH + dilation) |
pressure-overloaded LV (due to hypertension of stenosis)
(LVH + dilation (far R) is due to volume-overload (mitral or aortic insufficiency)) |
|
|
causes of Left-sided Heart Failure |
Ischemic heart disease hypertension aortic & mitral valvular disease cardiomyopathy |
|
|
clinical manifestations of Left-sided Heart Failure |
-pulmonary congestion & edema* -dyspnea & orthopnea* (supine SOB) -pleural effusion -atrial fibrillation & mural thrombi* -reduction of renal perfusion -hypoxic encephalopathy |
|
|
What does the reduction of renal perfusion lead to? |
activation of renin-angiotensin system pre-renal azotemia |
|
|
causes of Right-sided Heart Failure |
pulmonary hypertension (primary & secondary) pulmonary stenosis
*usually occurs due to L ventricular failure |
|
|
Clinical manifestations of Right-sided Heart Failure |
congestive hepatomegally peripheral edema pleural & pericardial effusions renal congestion hypoxic encephalopathy congestive splenomegaly bowel wall edema- ascites |
|
|
___________________ results from MI due to atherosclerotic coronary artery disease
*leading cause of death in developed nations |
Ischemic Heart Disease (IHD) |
|
|
Ischemic Heart Disease (IHD) syndromes: |
sudden cardiac death (SCD) MI angina pectoris chronic ischemic heart disease (CIHD) |
|
|
Acute coronary syndromes are usually caused by............ |
acute plaque change (plaque diruption) |
|
|
____________ are attacks of chest pain caused by transient myocardial ischemia (lasting <15 mins) & does NOT induce actual infarction
What are the 3 types? |
Angina Pectoris types; 1. stable angina 2. prinzmetal angina 3. unstable angina |
|
|
________ angina is brought on by inc workload & relieved by rest or NTG |
stable angina |
|
|
________ angina occurs at rest, caused by vasospasm, relieved by NTG |
Prinzmetal angina |
|
|
_______ angina causes pain that occurs w inc frequency & decreased effort, often at rest & inc in duration |
Unstable angina |
|
|
__________ is the ischemic necrosis of myocardium
What are the 2 types? |
myocardial infarction (MI) types;
1. transmural- Most common type, involves most of wall
2. subendocardial- involves inner 1/3rd - 1/2 of wall |
|
|
Sequence of events that occur in MI: |
1. plaque disruption--> 2. platelet adhesion & activation & tissue factor release---> 3. platelet activation causes granule release of ADP + TxA2 & inc Ca2+--> 4. tissue factor + Ca2+ activates coagulation cascade & ADP + TxA2 causes platelet aggregation--> 5. superimposed thrombus = infarction |
|
|
MI can also be induced by 4 other mechanisms |
vasospasm emboli disease of intramural vessels hemoglobinopathies |
|
|
In order for a stable plaque to cause angina, it must narrow the lumen by ______
Once the lumen is narrowed by _____ it can cause direct ischemia & possible infarction |
75% = angina
90% = ischemia (possible MI) |
|
|
When platelets aggregate on plaque what can occur? |
mural thrombus w/ variable obstruction emboli --> unstable angina or acute subendocardial MI or sudden death
or
occlusive thrombus--> acute transmural MI or death |
|
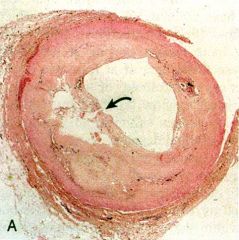
The arrow points to a ruptured atherosclerotic plaque. Is a superimposed thrombus present? |
NO |
|
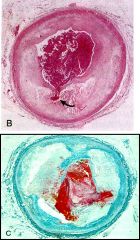
The arrow points to a ruptured atherosclerotic plaque (both images)
With or without a superimposed thrombus? |
WITH a superimposed thrombus
(bottom image has fibrin stained red) |
|
|
___________angina does not involve plaque distribution or thrombus but usually has stenosis |
stable angina |
|
|
__________angina the involved coronary artery would most likely exhibit plaque distribution & partially occlusive thrombus |
unstable angina |
|
|
_________ MI involves plaque distribution & occlusive thrombus |
Transmural MI |
|
|
_________MI involves plaque distribution & partially occlusive or occlusive w/ lysis thrombus |
Subenocardial MI |
|
|
_______ involves plaque distribution, stenosis, & occlusive or partially occlusive thromboemboli |
Sudden death |
|
|
After the onset of ischemia, cardiac myocytes will begin to be depleted of ATP they lose contractility w/i __ min lose 50% of ATP w/i ___ min lose 90& of ATP w/i ___ min thus irreversible cell injury occurs w/i 40 mins & microvascular injury w/i an hr |
contractility w/i 2 mins 50% ATP w/i 10 min 90% ATP w/i 40 min |
|
|
Myocardium distal to occlusion (area of risk) in the coronary artery is initially ___________ after 2 hrs the __________ will exhibit central ischemic necrosis After about 24 hrs the _________ will be necrosed |
initially ischemic
2 hrs- subendocardium exhibits necrosis
24 hrs- entire area of risk will be necrosed
(*IDENTIFY MI immediatly & restore blood supply to save as much myocardium as possible) |
|
|
Q: How much at risk myocardium is salvageable 2 hrs after occlusion of the coronary artery? |
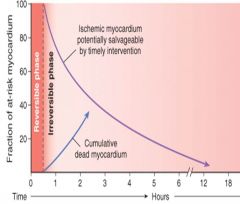
A: about 50% |
|
|
Most of the left ventricle infarctions result from occlusion of what arteries?
|
Left anterior descending (LAD)- 50% Right coronary artery (RCA)- 30% Left circumflex artery (LCX)- 20% |
|
|
Occlusion of the left anterior descending (LAD) artery causes MI in what part of the Left ventricle? |
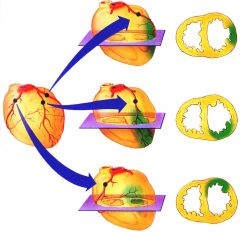
Anterior LV
(middle image) |
|
|
Occlusion of the right coronary artery (RCA) causes MI in what part of the LV? |
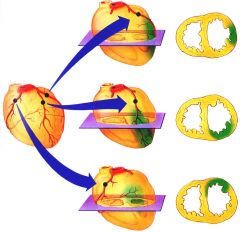
Posterior LV
(may also infarct across ventricular septum)
(bottom image) |
|
|
Occlusion of the left circumflex artery (LCX) causes MI in what part of the LV? |
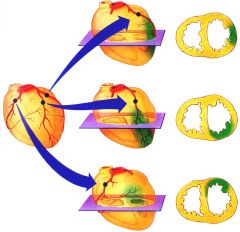
Lateral LV
(top image) |
|
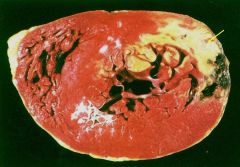
Post I Ant
The arrow point to an acute MI highlighted w TTC stain. What artery is likely responsible? |
arrow is pointing to MI involving the posterior & lateral wall of the LV (pale yellow area)
= Right coronary artery (posterior) or left circumflex artery (lateral)
(most likely LCX bc the black area = hemmorage is on the lateral wall) |
|
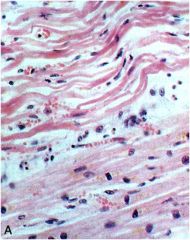
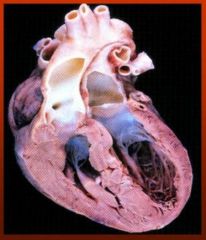
How old is the infarct in this tissue? why? |
1 day after infarct
- edema (white space) w/ early coagulative necrosis (pyknotic nuclei & hyperesosinophila), beginning neutrophil infiltration
|
|
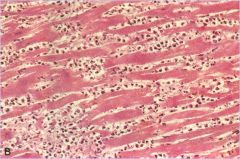
How old is the infarct in this tissue? why?
|
3-4 days after infarct
- coagulative necrosis w/ loss of nuclei & cross striations, heavy neutrophil infiltration |
|
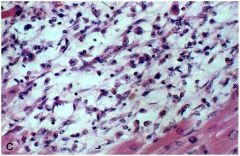
How old is the infarct in this tissue? Why? |
7-10 days after infarct
- macrophage infiltration, most of necrotic tissue has been phagocytized |
|
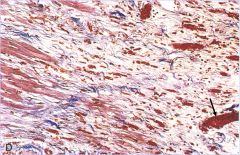
How old is the infarct in this tissue in this trichrome stain?
What is the arrow pointing to? |
3 weeks after infarct
- granulation tissue w/ prominent blood vessels are present, early collagen deposits are seen as wavy blue fibers
arrow= blood vessel |
|
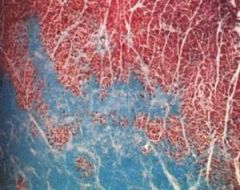
What does this image represent? |
healed MI (> 8 weeks after) (trichrome stain)
the infarcted tissue has been completely replaced by dense collagenous fibroconnective tissue = blue |
|
|
What are some of the compliations associated w dense collagenous fibroconnective tissue (scar tissue) depositing in the LV? |
-contractile dysfunction= arrhythmias -sudden cardiac death -ventricular rupture & cardiac tamponade -pericarditis -RV infarction -mural thrombus -ventricular aneurysm -papillary muscle dysfunction or rupture -chronic IHD |
|
|
What laboratory markers can be used to diagnose an acute MI?
(all can be detected w/i 3-12 hrs) |
Troponin I (cTnI) Troponin T (cTnT) CK-MB |
|
|
Which markers are the most accurate indicators? |
Troponin I & T
- specific to cardiac myocytes & remain elevated for 10-14 days |
|
|
CK-MB is (specific/not specific) & returns to normal w/i 72 hrs |
not specific * CK-MB is primarily in cardiac myocytes, HOWEVER a small amount is also present in skeletal muscle |
|
|
Systemic Hypertensive heart disease: Dx |
-LVH (on EKG & echo) (wall > 2 cm, heart > 500 g, enlarged myocytes) -hypertension (interstitial fibrosis)
(no other cardiovascular problems evident) |
|

What are these images showing? |
-increased wall thickness (L) -enlarged, rectangular nuclei (R)
= evidence of LVH |
|
|
*Systemic hypertensive heart disease predisposes pt to what complications? |
-CHF -sudden cardiac death -coronary atherosclerosis -atrial fibrillation |
|
|
What is NOT likely to occur in a patient w/ systemic hypertensive heart disease? |
RVH |
|
|
What is pulmonary hypertensive heart disease (Cor Pulmonale) characterized by? |
RVH RV dilation R heart failure (^resulting from pulmonary hypertension) |
|
|
What causes Cor Pulmonale? |
disorders of the lungs or pulmonary vasculature
(*RVH caused by LV failure or congenital heart diseases is NOT considered Cor pulmonale) |
|
|
Acute cor pulmonale is secondary to _________
Chronic cor pulmonale is secondary to _______ |
acute- massive PE
chronic- prolonged pressure overload |
|
|
Valvular heart disease:
What is the difference btwn stenosis & regurgitation? |
stenosis= valve cannot open completely
regurgitation= valve cannot close completely
(can occur together, both cause flow abnormalities--> murmurs/abnormal heart sounds) |
|
|
In general, is stenosis or regurgitation more common?
(more causes) |
regurgitation more common
|
|
|
Calcific aortic stenosis is the MOST common valvular abnormality, what causes it? |
normal wear & tear (or congenital issue) of the tricuspid valve
-may also be cause by a congenitally bicuspid aortic valve (2 cusps instead of 2= narrow) |
|
|
Calcific aortic stenosis: Dx |
-calcified nodules involving the cusps (echo) (commisures NOT fused) -LVH -angina -sudden death -syncope -CHF |
|
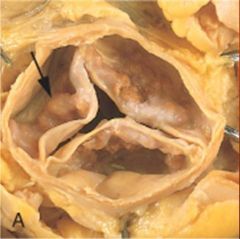
What is the arrow pointing at? |
calcified nodules w/i the tricuspid aortic valve
(=calcific aortic stenosis) |
|
|
Mitral annular calcification: Dx |
-stony hard calcified nodules in the mitral valve ring -women > 60 -pts w mitral valve prolapse |
|
|
Does mitral annular calcification cause stenosis or regurgitation?
|
BOTH
-if it interferes w/ fxn of valve ring= regurgitation
-if it impairs opening of mitral cusps= stenosis |
|
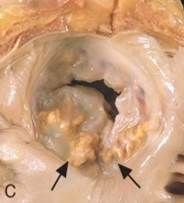
If thrombi forms on the ulcerated calcified nodules (arrows) what can occur? |
emboli |
|
|
Mitral valve prolapse (MVP) is due to...... |
myxomatous degeneration = deposition of proteoglycans in the mitral valve extracellular matrix
- associated w Marfan's syndrome--> fibrillin-1 mutation--> excess TGF-beta |
|
|
Why does mitral valve prolapse (MVP) cause a mid-systolic "click"? |
the valve leaflets are "floppy" & prolapse into the left atrium during systole = click
(this is the characteristic find in asymptomatic patients, may also cause systolic murmur) |
|
|
What are the possible complications of MVP? |
-mitral regurgitation -infective endocarditis -stroke -arrhythmias |
|
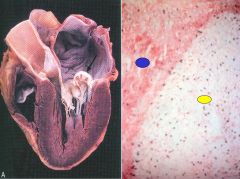
What is the black arrow pointing to? blue dot? yellow dot? |
black arrow: hooded posterior leaflet that is prolapsed into the left atrium (chordae tendinae is also enlongated & LA dilated)
blue dot: fibrosa layer of mitral valve leaflet w/ myxomatous degeneration
yellow dot: spongiosa layer of the valve leaflet, stroma of this layer has very loose appearance
=MVP |
|
|
Rheumatic fever may occur as a hypersensitivity reaction a few weeks after a ______________ infection |
group A strep
(response against streptococal M proteins--> cross rxn w self-proteins) |
|
|
Rheumatic fever: morphologic findings |
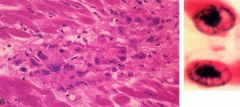
pancarditis Aschoff bodies (L img) Anitschkow cells (R img) vegetations (verrucae) |
|
|
What are Anitschkow cells? |
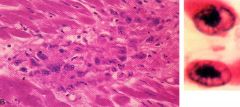
(right image) activated macrophages w/ prominent chromocenters
*pathognomonic for rheumatic fever |
|
|
Rheumatic fever causes carditis and may eventually progress to ________________ |
Rheumatic heart disease (RHD)- deforming fibrotic valvular disease |
|
|
Rheumatic heart disease (RHD): clinical manifestations |
murmurs hypertrophy/dilation CHF atrial fibrillation thromboemboli infective endocarditis |
|
|
Rheumatic heart disease (RHD): morphological findings |
fibrosis w/ thickened leaflets commissural fusion shortening, thickening & fusion of cords |
|
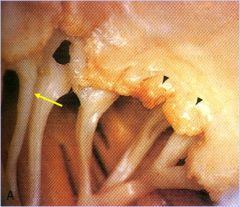
what pathology?
what are the black arrows pointing to?
yellow arrow? |
Acute rheumatic mitral valvulitis superimposed on chronic rheumatic heard disease (RHD)
black arrows: small vegitations composed principally of verrucae (fibrin)
yellow arrow: fused chordae tendineae (from previous episodes of RF, also see fibrous thickening of leaflets due to this) |
|
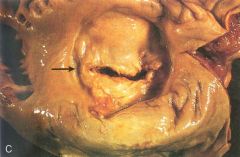
what pathology?
what is the arrow pointing at? |
Rheumatic heart disease (mitral valve stenosis)
black arrow: commissural fusion--> fibrous tissue bridge across commissure fusing leaflets together (characteristic to RHD)
-valve leaflets thickened from fibrosis--> severly stenotic valve w/ small slit opening = "fish mouth" stenosis |
|
|
Infective endocarditis (valve infection due to microbes) leads to the formation of ___________________ |
vegetations |
|
|
Vegetations most commonly involve what valves?
What about in IV drug users? |
MC= aortic & mitral valves
IV drug users= tricuspid valves |
|
|
What is the difference btwn acute & subacute infective endocarditis? |
acute- destructive infection involves previously normal valve highly virulent organism death w/i days-weeks
subacute- less destructive involves deformed valve low virulence most patients recover |
|
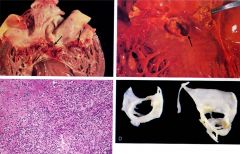
which image is acute & subacute?
Which valves are involved?
What are the arrows pointing to? |
upper left= subacute mitral valve arrow- vegetations
upper right= acute aortic valve arrow- ring abscess |
|
|
Why are ring abscesses a concerning complication of acute infective endocarditis? |
They can penetrate into the ventricular septum & cause conduction disturbances & arrhythmias or can pentrate into left ventricle free wall and cause cardiac tamponade |
|
|
If acute infective endocarditis in untreated it will result in extensive destruction of the leaflets, creating _____________, which will result in ________ |
large fenestration (holes in leaflets) ^ which will result in severe mitral regurgitation |
|
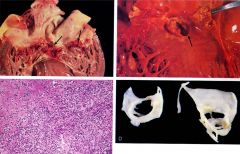
What would the histologic section of a vegetation reveal? (bottom left image) |
majority of cells = neutrophils eosinophilic material= fibrin
(gram stain would likely reveal bacterial cause of infection)
|
|
|
What factors predispose to infective endocarditis? |
-abnormal/prosthetic valve -neutropenia, immunosuppressed, diabetes -IV drug abuse, dental/surgical procedure |
|
|
In pts w/ normal valves the most common (MC) organism in infective endocarditis is ____________ |
staph. aureus
(normal on skin, IV drug use/surgicial procedure allows organism into body) |
|
|
In pts w/ abnormal valves the MC infective organism is ____________ |
strep. viridans |
|
|
In pts w/ prosthetic valves, what is the MC organism? |
staph. epidermis
(sometimes give pts prophylactic antibiotics after valve replasement to prevent this) |
|
|
What are the symptoms of infective endocarditis? |
fever murmurs (esp changing or recent onset regurgitation murmurs) petechiae roth spots |
|
|
What can infective endocarditis lead to? (complications) |
-regurgitation or stenosis of valve -CHF -ring abscess -prosthetic valve leak -septic emboli -glomerulonephritis |
|
|
Nonbacterial thrombotic endocarditis (NBTE) is strongly assoiciated w ______________________ |
mucinous adenocarcinoma
(esp in debilitated patients w/cancer, sepsis (DIC))
(NOT bacterial infection) |
|
|
NBTE is characterized by, |
-small thrombotic vegetations on valves (along line of closure of valve) -sterile -noninflammatory -nondestructive -hypercoaguable state (due to mucin)
|
|
|
Why are the vegetations in NBTE dangerous? |
they are loosely attached thrombotic vegegations--> may dissociate & cause emboli--> infarct etc |
|
What pathology? Describe the vegetations on this mitral valve. |
Nonbacterial thrombotic endocarditis (NBTE)
-small fibrin vegetations along the line of closure of the mitral valve |
|
|
What would you expect to see in a histological section of a valve cusp w/ NBTE? |
-loosely attached thrombus (arrow) -fibrin thrombus
(few or no inflammatory cells & microorganisms) |
|
|
Libman-Sacks endocarditis is associated w/ what pathology?
What valves does it typically involve? |
SLE (also called Endocarditis of SLE)
valvulitis of mitral & tricuspid valves |
|
|
How is endocarditis of SLE (Libman-Sacks) unique? |
-vegetations are on BOTH upper & undersurface of valves** -hematoxylin bodies can sometime be seen
-vegetations are small, sterile, & mostly fibrin
-can lead to subsequent fibrosis & deformity similar to RHD
|
|
|
What are hematoxylin bodies? |
bare nuclei coated w/ antinuclear antibodies (ANAs)
(=round/oval bodies that stain blue) |
|

Which pathologies are these? Why?
|
Left- Rheumatic fever= very small vegetations along line closure of valve, inflammatory
Right- Infective Endocarditis= large vegetations, irregular & destructive, involves leaflets & chordae tendineae |
|
Which pathologies are these? Why? |
Left- Nonbacterial thrombotic endocarditis= small vegetation along line closure of valve, sterile, non-inflammatory, non-destructive, loosely attached
Right- Libman-Sacks (SLE) endocarditis= small vegetations present on upper & under surface |
|
|
Carcinoid syndrome is characterized by flushing, cramp, diarrhea, asthma due to GI carcinoid tumor w/ hepatic metastases. What can it lead to? |
Carcinoid heart disease
(mostly due to tumor secretion of serotonin) |
|
|
Carcinoid heart disease causes thickening of the endocardium in the ________________, due to myofibroblast proliferation & deposition of collagen & acid mucopolysaccharides |
-thickening of endocardium of right atrium & ventricle, pulmonary & tricuspid valves |
|
|
Carcinoid heart disease also causes right-sided heart lesions. The severity of the lesions can be correlated to plasma levels of _______________
Lesions may progress to right-sided (tricuspid) valvular insufficiency or stenosis
(bc serotonin is inactivated in lungs- doesn't cause left- problems) |
serotonin
(carcinoid tumors also secrete histamine, NE, etc but most symptoms are due to serotonin) |
|

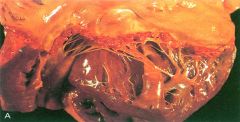
What does this image show? |
Carcinoid heart disease
endocardial thickening extends down to tricuspid leaflets--> tricuspid insufficiency |
|

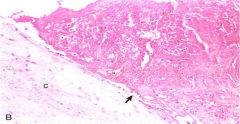
What pathology does this Movat stain show? |
Carcinoid heart disease
-black elastic tissue -blue acid mucopolysaccharides abundant in the endocardium
endocardial plaque-like thickenings = carcinoid plaques = myofibroblasts + acid mucopolysaccharides (or collagen) |
|
|
Cardiomyopathies (heart muscle diseases) may be primary (genetic/aquired) or secondary (resulting from another initial pathology).
What are the 3 types? |
-dilated cardiomyopathy -hypertrophic cardiomyopathy -restrictive cardiomyopathy |
|
|
_________ cardiomyopathy: -enlarged, globoid, "flabby" heart -all chambers involved -often w/ mural thrombi -reduced ejection fraction (25%, normal- 50%) -death w/i 2 yrs -viral (coxsackie B), toxin, nutritional deficiency
|
Dilated cardiomyopathy (DCM) |
|
|
What occurs in dilated cardiomyopathies (DCM)? |
progressive dilation (of all chambers)--> contractile dysfunction & hypertrophy--> progressive CHF--> death |
|
|
What are the main toxins that cause dilated cardiomyopathies?
what nutrient deficiency? |
toxins: alcohol chemo drugs cobalt (beer foaming agent)
nutrient: thiamine (worsened by alcohol abuse) |
|
|
Dilated cardiomyopathy (DCM) may also be cause by excess iron, catecholamines, or by excess ____________ after pregnancy |
antiangiogenic mediators
(prolactin cleavage products that reduce blood vessels so that less blood is lost during delivery, these may cause MI)
(iron overload= inc free radicals) |
|
|
What are some of the genetic causes of DCM? |
mutations in; cytoskeleton genes (dystrophin- muscular dystrophy) mitochondrial genes sarcomere genes (titin) nuclear envelope genes (lamin A/C) |
|
|
_____________ cardiomyopathy; -impaired diastolic filling -hypercontractibility during systole -systolic anterior motion "SAM" -left ventricular outflow tract (LVOT) obstruction -decreased CO *due to mutations in sarcomere genes* |
hypertrophic cardiomyopathy (HCM) |
|
|
What is systolic anterior motion (SAM)? |
anterior mitral leaflet moves anteriorly during the suptum during systole= SAM
this causes transient LVOT obstruction--> decreasing cardiac output |
|
|
Clinical features of hypertrophic cardiomyopathy (HCM)? |
-angina, arrhythmias, sudden death -atrial fibrillation w/ mural thrombus formation -systolic murmur, CHF -infective endocarditis of the mitral valve |
|
|
Morphologic Features of HCM |

(trichrome stain) -myocyte hypertrophy -myofiber disarray -fibrosis (secondary to loss of some myocytes due to ischemia caused by the hypertrophied myocytes) |
|
|
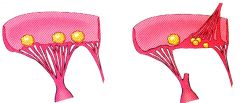
Gross features of HCM |
asymmetrical septal hypertrophy (ratio > 1.3) -both septum & LV hypertrophied, but septum more--> causes LV chamber to be smaller (banana shaped)--> impaired diastolic filling (--> this causes LA to be dilated)
|
|
|
____________ cardiomyopathy; -decreased ventricular compliance--> impaired diastolic filling -dilated atria (ventricles usually normal)
|
Restrictive Cardiomyopathy |
|
|
Restrictive Cardiomyopathy my be idiopathic or associated w; |
radiation fibrosis amyloidosis sarcoidosis metastatic tumor storage diseases enomyocardial fibrosis loeffler endomyocarditis endocardial fibroelastosis |
|
|
Myocarditis, or injury of myocytes, manifests as; fever fatigue dyspnea palpitations precordial pain
What can it progress to? |
heart failure- systolic mumur- arrhythmias Dilated cardiomyopathy |
|
|
Myocarditis is caused by |
infection: Viral infections (coxsachie A & V --> DCM) (^most common) Chagas disease (trypanosoma cruzi)
immune response: hypersensitivity
unknown: Giant cell myocarditis
|
|
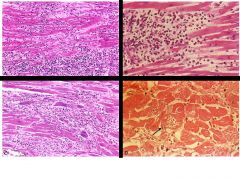
Which image shows lymphocytic myocarditis? |
upper left
---> viral myocarditis manifestation ---> mostly lymphocytes w/ focal necrosis |
|
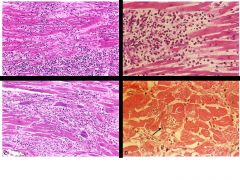
Which image shows hypersensitivity myocarditis? |
upper right
---> numerous eosinophils w/i inflammatory infiltrate ---> hypersensitivity usually a drug rxn |
|
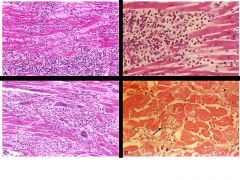
Which image shows giant cell myocarditis? |
lower left
---> scattered giant cells w/ extensive loss of myocytes (necrosis)
---> giant cell myocarditis has a poor prognosis |
|
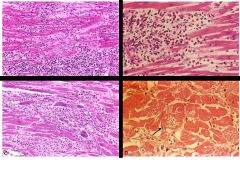
Which image shows Chagas disease? |
lower right
---> arrow pointing to myofiber distended w/ trypanosomes (parasites)
---> usually in South America |
|
|
Pericarditis is usually secondary to cardiac, thoracic, or systemic disorders. What are the different types? |
serous fibrinous & serofibrinous purulent (suppurative) hemorrhagic constrictive
|
|
|
_______ pericarditis; -mild inflammation -serous exudate (watery effusion w/ few cells) -RF, SLE, uremia, viral infection, tumors (w/o adhesions) |
serous pericarditis |
|
|
__________ Pericarditis; -inflammation -acute MI, postinfarction (Dressler) syndrome, radiation, heart surgery, trauma, RF, SLE, uremia -loud pericardial friction rub -possible adhesions (may heal w/o)
|
Fibrinous & Serofibrinous Pericarditis
fibrinous- dry exudate or serofibrinous- fibrin & fluid exudate |
|
What type of pericarditis? |
Fibrinous pericarditis
-"shaggy" fibrinous exudate coating epicardial surface of heart |
|
|
___________ pericarditis; -acute inflammation -purulent exudate -bacterial infection -scarring--> constrictive pericarditis |
Purulent (suppurative) pericarditis |
|
|
____________ pericarditis; -fibrinous or suppurative exudate mixed w/ blood -malignancy, bacterial infections, bleeding diathesis, TB, heart surgery |
Hemorrhagic pericarditis |
|
|
____________pericarditis; -hx of suppurative, hemorrhagic or caseous pericarditis -heart encased by thick fibrous tissue -resembles restrictive cardiomyopathy -encasement prevents ventricle expansion during diastole -Tx by pericardiectomy (remove thick tissue) |
Constrictive pericarditis |
|
What type of pericarditis? |
constrictive pericarditis |
|
|
Cardiac tumors are commonly benign except for __________
which is the most common benign tumor (MC of all cardiac tumors) |
Angiosarcoma = malignant
Myxoma= benign * overall MC |
|
|
Cardiac tumors are also metastatic from where? |
LUNG & breast carcinomas melanomas leukemias lymphomas
--> result in restrictive cardiomyopathy |
|
|
Myxomas are usually located in the (atria/ventricles) |
atria |
|
|
Myxomas can be diagnosed w/ echo. What are the clinical manifestations? |
-obstruction of mitral valve orifice -embolization--> stroke -fever & malaise due to secretion of IL-6 (possible Carney complex) |
|
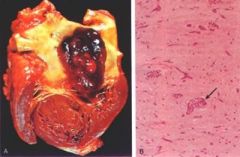
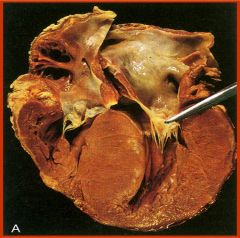
Large myxoma w/i LA
what are the morphologic features that are characteristic? |
morphology: -gelatinous sessile or polypoid masses -stellate* & other cells embedded in myxoid extracellular matrix = loose myxoid stoma w abundant acid mucopolysaccharide deposition
arrow= abnormal vessel- characteristic tumor feature |
|
|
___________ is the most common type of heart disease in children |
congenital heart disease
(faulty embryogenesis during wks 3-8) |
|
|
Most mutated genes involved in congenital heart defects encode for _____________
What defects does this usually lead to? |
transcription factor genes
usually Atrial & ventricular septal defects |
|
|
Another congenital heart disease ___________, results from mutated fibrillin gene. What does this cause? |
Marfans
mitral valve prolapse--> myxomatous degeneration (valve abnormality) |
|
|
Gene mutations in congenital heart disease are often multifactoral with environmental causes such as, |
tetratogens (alcohol, retinoic acid, dilantin during preg) rubella infection |
|
|
3 types of congenital heart disease |
1. R --> L shunt (cyanotic congenital heart disease)
2. L--> R shunt (Eisenmenger syndrome)
3. Obstruction (obstructive congenital disease) |
|
|
Overall most common heart defect? |
Ventricular septal defect
(2nd most atrial septal defect) |
|
|
What defect? L--> R shunt abnormal opening in atrial septum usually isolated defect systolic murmur usually asymptomatic until adulthood low mortality rate |
Atrial septal defect (ASD) |
|
|
What is the most common type of atrial septal defect?
Possible complications? |
Ostum secundum (MC) (hole in middle of septum)
complications: irreversible pulmonary htn right sided heart failure paradoxical embolization |
|
|
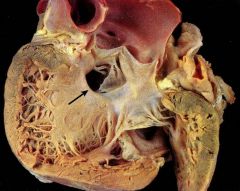
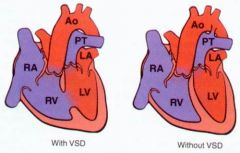
What defect? L --> R shunt incomplete closure of ventricular septum most common congenital cardiac defect often assoc w other cardiac defects systolic murmur Severity varies w/ size of hole |
Ventricular septal defect (VSD) |
|
|
what is the most common type of ventricular septal defect?
Complications of VSD? |
defect involving membranous septum
complications: irreversible pulmonary htn late cyanosis right sided heart failure |
|
|
What defect? L --> R shunt (aorta--> pulmonary artery) ductus arteriosus remains patent after birth pressure in aorta > pulmonary artery usually isolated continuous harsh murmur (during systole & diastole) severity varies w. size
|
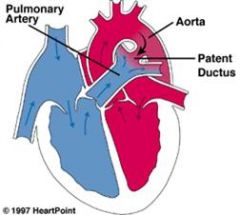
Patent ductus arteriosus |
|
|
Complications of patent ductus arteriosus (PDA)
tx? |
complications: irreversible pulmonary hypertension late cyanosis right-sided heart failure
tx: indomethacin (sometimes works) |
|
|
What defect? -R ---> L shunt -anterior displacement of aorticopulmonary septum--> unequal division of truncus arteriosus & bulbus cordis -1. overriding aorta, 2. VSD, 3. subpulmonary stenosis, 4. RVH -cyanotic -Severity varies depending on degree of subpulmonary stenosi (pink= mild)
|
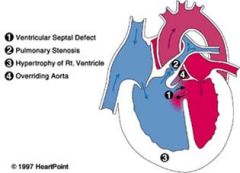
Tetralogy of fallot |
|
|
What defect is beneficial & may be surgically created in patients w tetralogy of fallot? |
patent ductus arteriosus
(give prostaglandins after birth to keep open, allows so blood to get oxygen |
|
|
What defect? -aorta comes from RV & pulmonary artery from LV (aorticopulmonary septum not normal) -incompatible w/ life w/o another shunt -cyanotic |
Transposition of Great Arteries |
|
|
What shunts make transposition of Great arteries viable? |
VSD (membranous) patent foramen ovale PDA
(or create shunt via septostomy or fix w arterial-switch operation) |
|
|
What defect? -narrowing of aortic arch proximal to PDA -systolic murmur & continous murmur -cyanosis in lower half of body -usually requires nonatal surgery |
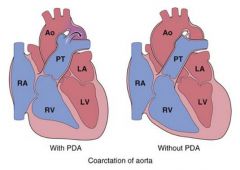
coarctation of the aorta w/ PDA (infantile) |
|
|
What defect? -narrowing of aortic arch opposite closed ductus arteriosus -systolic murmur -LVH -notching of ribs on CXR -hypertension of upper extremities -low BP in lower extremities -may be asymptomatic -tx w surgery |
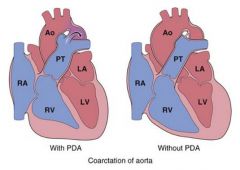
coarctation of the aorta w/o PDA (adult) |
|
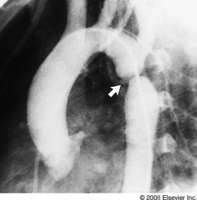
What does this angiogram show? |
coarctation of the aorta |
|
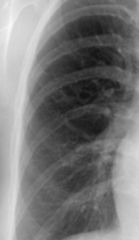
What does this CXR show? |
notching of the ribs --> coarctation of the aorta, adult type
(enlarged intercostal arteries = collaterals to bypass coarctation obstruction--> erosed undersurface of ribs = notching) |

