![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
114 Cards in this Set
- Front
- Back
|
characteristic functional group in a carboxylic acid
|
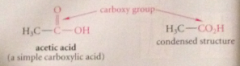
carboxy group
|
|
|
characteristic functional group in a sulfonic acid
|

sulfonic acid group
|
|
|
A carboxylic acid is named by adding the suffix
|
ic and the word acid to the prefix for the appropriate group
|
|
|
dicarboxylic acids
|
carboxylic acids w 2 carboxy groups
|
|
|
Successive dicarboxylic acids "(oh my such good apple pie)"
|
oxalic, malonic, succinic, glutaric, adipic, pimelic
|
|
|
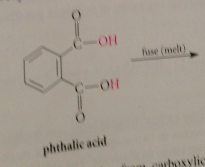
phthalic acid
|
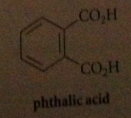
aromatic dicarboxylic acid
|
|
|
a carboxylic acid is named by dropping
|
final e from hydrocarbon w same number C atoms & adding suffix oic acid
|
|
|
Is the final e dropped in naming dicarboxylic acids?
|
No
|
|
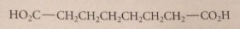
Name this compound
|
octanedioic acid
|
|
|
When a carboxylic acid is derived from a cyclic hydrocarbon, the suffix
|
carboxylic acid are added to name of hydrocarbon
|
|
|
The principal chain in substituted carboxylic acids is numbered by assigning the number 1 to
|
the carbonyl carbon
|
|
|
In carboxylic acids derived from cyclic hydrocarbons, numbering begins @
|
the ring carbon bearing the carboxy group
|
|
|
Priority for citation as principal group
|

|
|
|
HO2C-CH2 substituent
|
carboxymethyl group
|
|
|
Carboxylic acid geometry
|
trigonal
|
|
|
C-O (Carboxylate oxygen) bond is ___ than the C-O bond in an alcohol or ether: why?
|
shorter (In an acid it is sp2-sp3 hybridized, in alcohol or ether sp3-sp3 single bond)
|
|
|
The carboxylic acids of lower mlclr mass are
|
high boiling liquids w acrid, piercing odors & higher bp than other organic cmpds of same mlclr mass & shape
|
|
|
Why do carboxylic acids have high bp?
|
polarity & strong hydrogen bonds formed
|
|
|
In solid state & sometimes in both gas phase & solution, carboxylic acids exist as
|

H-bonded dimers w large equilibrium constants
|
|
|
Many aromatic & dicarboxlyic acids are
|
solids
|
|
|
Simpler carboxylic acids are
|
soluble in h2o as expected from H-bonding capabilities (unbranched carb acids below pentanoic acid are miscible w h2o)
|
|
|
C=O stretching absorption
|
1710 cm-1 (benzoic acid: 1680)
|
|
|
carb acid OH stretch absorption
|
broader than OH in alcohol or phenol, covers 2400-3600
|
|
|
a-protons of carb acids NMR absorption
|
2-2.5 ppm
|
|
|
OH proton resonance
|
9-13 ppm region, often broad
|
|
|
How to distinguish acid proton from aldehydic proton?
|
acid proton like alcohol rapidly exchanges w D2O
|
|
|
diff btwn carb acid & aldehyde/ketone 13C NMR & why
|
carbonyl carbon of acid has somewhat smaller chem shift than aldehyde/ketone bc of shielding effects of unshared e pairs on carboxylate O
|
|
|
Why are carb acids acidic?
|
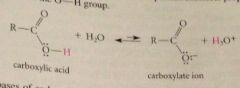
Ionization of the OH group
|
|
|
carboxylate ions
|
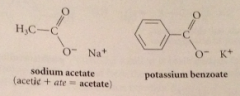
conjugate bases of carboxylic acids: replace ic with ate
|
|
|
What is most acidic, alcohol, phenol or CA?
|
ROH < Ph-OH < CA
|
|
|
Why is CA so acidic?
|
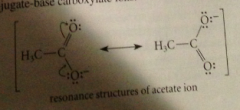
polar effect of carbonyl gorup (sp2 hybridized atoms, so partial pos charge on carbonyl C, presence of O more electroneg than phenyl or alkyl) stabilizes charge in carboxylate ion = enhanced acidity + resonance stabilization of carboxylate ion
|
|
|
halogen substitution within the alkyl group of a carboxylic acid ___ acidity by __
|
enhances - polar effect
|
|
|
Sulfonic acids are much ___ than comparably substituted carboxylic acids
|
stronger
|
|
|
Why is sulfonic acid so acidic?
|
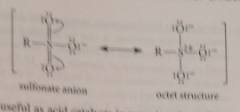
Sulfur's high oxidation state: octet structure indicates positive charge on sulfur: stabilizes neg charge on oxygens
|
|
|
Sulfonic acids are __ soluble than most inorganic acids
|
more
|
|
|
Sulfuric acid is ___ soluble in benzene and toluene
|
insoluble
|
|
|
Many carboxylic acids of moderate mm are ___ in water while alkali metal salts are ___
|
insoluble -- more soluble
|
|
|
Many water-insoluble carboxylic acids dissolve in solutions of alkali metal hydroxides NaOH, KOH because
|

the insoluble acids are converted completely into their soluble salts
|
|
|
Henderson-Hasselbach equation
|
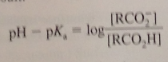
|
|
|
A typical CA can be separated from mixtures with other
|
water-insoluble, nonacidic substances by extraction w NaOH, Na2CO3, or NaHCO3
|
|
|
After separating the basic aq. solution, it can be acidified w a strong acid to yield CA, which may be
|
isolated by filtration or extraction w organic solvents
|
|
|
CA can also be separated by extraction w 5% NaHCO3 if the phenol is not unusually
|
acidic
|
|
|
Why does the phenol remain largely unionized and thus insoluble in an aq. solution w a pH of 8.5?
|
the pka of a typical phenol is about 10
|
|
|
The carbonyl oxygens of acids like ald/ket are
|

weakly basic
|
|
|
Why does protonation of an acid on the carbonyl oxygen occur?
|
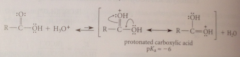
A resonance stabilized cation is formed
|
|
|
Why is protonation on the carboxylate oxygen much less favorable?
|
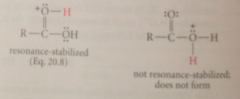
It does not give a resonance-stabilized cation & positive charge on oxygen destabilized by polar effect of carbonyl group
|
|
|
Fatty acids
|
CA w long, unbranched C chains
|
|
|
Saponification
|
Liberation of fatty acids form fats and oils by a hydrolytic process
|
|
|
Some fatty acids contain C=C that are
|
cis (trans is rare in nature)
|
|
|
soaps
|
sodium & potassium salts of fatty acids
|
|
|
detergent
|
any substance used for cleaning an object by immersing it in a liquid solution
|
|
|
surfactants
|
mlcs w polar head group & hydrocarbon tail not readily solvated by h2o
|
|
|
In a soap what is polar & hydrocarbon?
|
polar head is carboxylate anion, hydrocarbon tail is C chain
|
|
|
anionic surfactants
|
surfactants w anionic polar head group
|
|
|
cationic surfactants
|
surfactant with cationic polar head group
|
|
|
critical micelle conc
|
concentration of surfactant at which mlcs spontaneously form micelles: spherical aggregates
|
|
|
How do detergents work?
|
dirt associates w hydrocarbon on interior of micelle & is incorporated into micellar aggregate, thus lifted away from surface of fabric carried into solution
|
|
|
when a bacterial cell is exposed to a solution w a surfactant, phospholipids of cell membrane tend to
|
associate w the surfactant, in some cases can disrupt the membrane enough that the cell can no longer function & dies
|
|
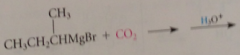
|

typically run by pouring ether solution of grignard reagent over crushed dry ice
|
|
|
Mechanism preparation CA
|
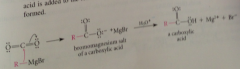
Addition of grignard reagent to CO2 gives bromomagnesium salt of CA & aq. acid added => free CA
|
|
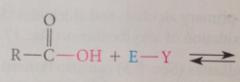
substitution at the carbonyl carbon: most typical rxn @ carbonyl group
|
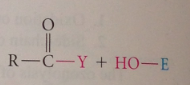
|
|
|
rxn of carbonyl O w an electrophile (rxn of carbonyl O as a base)
|
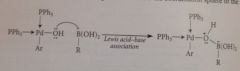
|
|
|
The reactions of nuc at the carbonyl carbons are catalyzed by
|
reactions of acids at the carbonyl oxygen
|
|
|
rxn @ carboxylate oxygen: ionization of CAs
|
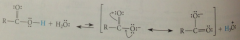
|
|
|
reaction of carboxylate oxygen as nuc
|
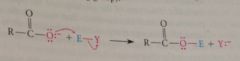
|
|
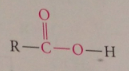
decarboxylation
|
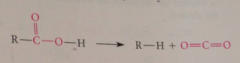
loss of carboxy group as CO2
|
|
|
Esters
|
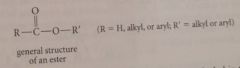
carboxylic acid derivatives
|
|
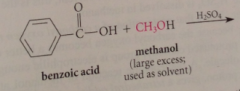
acid catalyzed esterification/ Fischer esterification
|
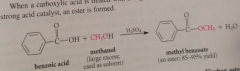
small equilibrium constant
|
|
|
Fischer rxn driven to completion by
|
using reactant alcohol as solvent (driven toward ester prod)
|
|
|
Can acid-catalyzed esterification be applied to the synthesis of esters from phenols or tertiary alcohols?
|
No: tertiary alcohols undergo dehydration & other rxns under acidic conditions & equil. constants for esterification of phenols much less favorable than those for alcohols
|
|
|
Mechanism of acid-catalyzed esterification
|
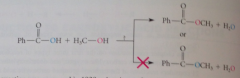
Substitution of OH at the carbonyl group of the acid by the oxygen of the alcohol
|
|
|
Mechanism of acid-catalyzed esterification step 1
|
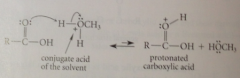
|
|
|
protonation of a carbonyl O makes carbonyl C more
|
electrophilic
|
|
|
formation of tetrahedral addition intermediate
|
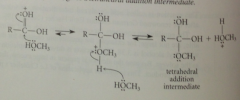
essentially same as acid-catalyzed rxn of alcohol w protonated aldehyde or ketone to form hemiacetal
|
|
|
tetrahedral addition intermediate reacts further
|

protonated then loses water to give conjugate acid of ester
|
|
|
Loss of a proton gives
|

ester product, regenerates acid catalyst
|
|
|
general substitution rxns of CAs
|
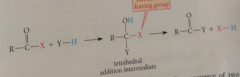
|
|
|
Why don't ald/ket undergo sub @ carbonyl C?
|
After nuc reacts @ carbonyl C, neither groups attached can act as LG bc would be expelled as H- or R- either of which is a very strong base
|
|
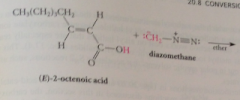
When a carboxylic acid is treated with diazomethane in ether solution
|
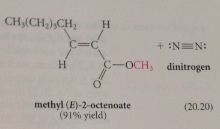
it is rapidly converted into its methyl ester
|
|
|
ester formation mechanism
|
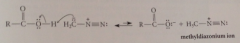
protonation of diazomethane by CA gives methyldiazonium ion
|
|
|
is dinitrogen a good or bad leaving group?
|
GREAT
|
|
|
Sn2 reaction of methyl-diazonium ion with carboxylate oxygen
|

results in displacement of N2 & formation of ester
|
|
|
Are carboxylate ions basic?
|
Less nuc than alkoxides or phenoxides but react w reactive alkylating agents
|
|
|
CA is required for reaction with diazomethane bc
|
protonation of diazomethane is first step of reaction
|
|
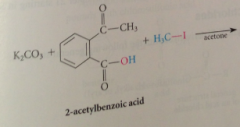
typically carried out in PA solvents that accelerate SN2 like acetone
|
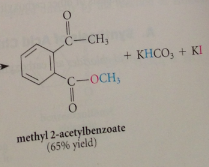
Sn2 rxn in which carboxylate ion reacts as nuc w alkyl halide - best on alkyl halides that are especially reactive in sn2 rxns such as methyl iodide & benzylic /allylic halides
|
|
|
Acid chlorides
|
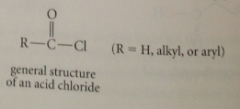
|
|
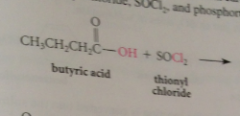
|
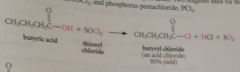
|
|
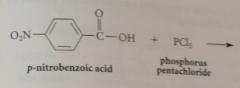
|

|
|

|
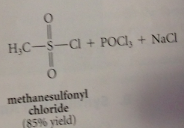
|
|
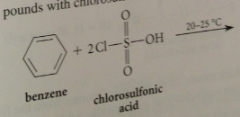
|
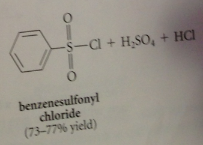
|
|
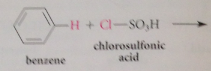
|
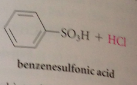
|
|
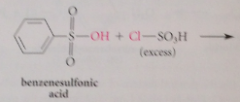
|
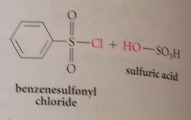
|
|
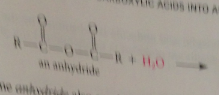
|
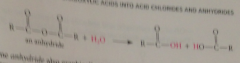
|
|
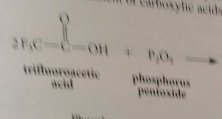
|
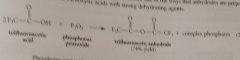
|
|
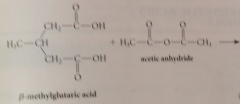
|
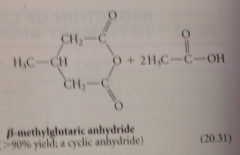
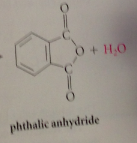
cyclic anhydride
|
|
|
P2O5
|
inorganic anhydride used to form anhydrides of CAs
|
|
|
|
|
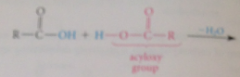
|
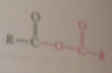
|
|
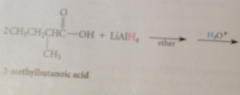
|
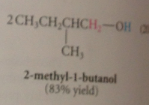
|
|
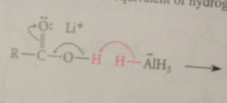
Mechanism CA reduction
|
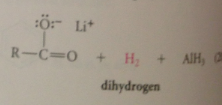
LiALH4 reacts w acidic H of CA to give Li salt of CA + 1 equiv H2
|
|
|
What species is reduced?
|
The lithium salt of the CA
|
|
|
Mechanism CA reduction 2
|
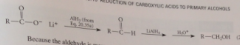
AlH3 reduced carboxylate ion into an aldehyde, which is reduced to give after protonolysis, the primary alcohol
|
|
|
The aldehyde cannot be isolated because
|
it is more reactive than the carboxylate salt
|
|
|
Net substitution followed by net addition
|
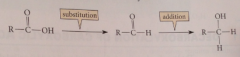
|
|
|
Does NaBH4 reduce CA?
|
No
|
|
|
Decarboxylation
|

loss of CO2 from a carboxylic acid
|
|
|
b-keto acids
|
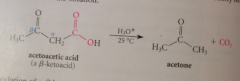
CA w keto group in B position: readily decarboxylate @ room temp in acidic solution
|
|
|
Enol intermediate formed by intermlcr transfer from CA group to carbonyl O atom of ketone
|
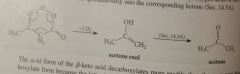
enol transformed spontaneously into corresponding ketone
|
|
|
The acid form of the B-keto acid decarboxylates more readily than the conjugate base carboxylate form because
|
base has no acidic proton that can be donated to the B-carbonyl oxygen (promotes its own removal)
|
|
|
Malonic acid: readily decarboxylates upon heating in acidic solution
|
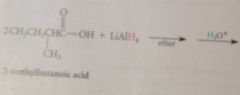
requires heating so acids can be isolated @ room temp
|
|
|
Carbonic acid
|
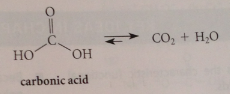
unstable, decarboxylates in acidic solution to co2 + h2o
|
|
|
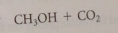
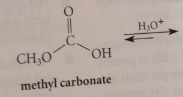
any carbonic acid derivative w a free carboxylic acid group will decarboxylate under acidic conditions
|
|
|
Under basic conditions, carbonic acid & derivatives
|
exist as carboxylate salts & do not decarboxylate
|
|
|
Carbonic acid diesters & diamides are
|
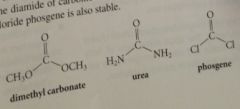
stable
|

