![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
Define toxicity |
the consequence of a deleterious (harmful or undesirable) effect of asubstance in a biological system. |
|
|
What is a toxicant? |
A toxicant is any chemical, physical or biological agent which is harmful to livingorganisms as a result of physical or chemical interaction. |
|
|
Source, pathway and receptor |
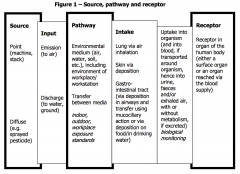
|
|
|
What are the routes of occupational exposure to chemicals |
Inhalation Skin contact Ingestion |
|
|
Categorise exposure duration acute repeated - sub acute - sub chronic - chronic |
Acute - single episode (usually a few hours) Repeated (multiple exposures ie daily) Sub-acute - up to 30 days Sub-chronic - 30-90 days chronic - over 90 days (includes lifetime) |
|
|
Manifestations of toxicity |
Toxic effects can be: Immediate (rapidly after exposure narcosis after solvent exposure) Delayed (some time after exposure as with cancer,fibrosis or delayed neuropathy with organophosphorus pesticides) Reversible (narcosis, or damage to an organ with regenerative capacity) Irreversible (loss of neurons in the brain, development of a tumour) LD50 - identify dose that caused 50% of the animals to die Oganophosphorus pesticides causevarious signs including salivation, tremors and convulsions, which increase inseverity with dose. Also, animal studies generally include a pathologicalexamination (necropsy), where tissues and organs are examined grossly andmicroscopically. These examinations can reveal changes in tissues, such as celldeath (necrosis) or increased cell replication (hyperplasia), which are oftoxicological significance. There may also be observable changes in biochemicalparameters, for instance an increase in the blood of enzymes which have beenreleased from damaged cells (e.g. lactate dehydrogenase) or changes in hormonelevels, which correlate with the underlying toxicity. |
|
|
Dose-effect Dose-response relationships Quantal/stochastic effect |
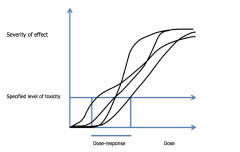
The dose-effect relationship can show how the severity of an effect - which can beminor psychological, biochemical or pharmacological perturbations or (usually athigher doses) pathological change, and, in extreme cases death - varies with dose.It can be related to the individual or, if the inter-individual variation is relativelylow, to a population The dose-response relationship shows the relationship between a given level of atoxic effect and the received dose in a group of individuals (a population). This isapplied when examining the likelihood (in the individual) or frequency (in apopulation) with which a given level of response is seen Quantal/stochastic - end-point is all or nothing response such as cancer or death as well as a predetermined level of response when there is a graded response To convert a population dose-effect relationship to adose-response relationship a specific level of effect (in major hazards toxicity a‘specified level of toxicity’ – SLOT) is chosen and the inter-individual variation inthe dose achieving that level of effect is plotted It can be applied when the end-point is an all or nothing response (a quantalor stochastic effect such as death or cancer), as well as at a selected fixed endpoint (e.g. ability to get up and walk) when there is a graded response, such asthat associated with solvent induced behavioural effects (in the case if narcosis,from effects only detected by psychological testing through incoherence andincoordination, and, if the dose is high enough, anaesthesia). The presumptions underlying the dose-effect and dose-response relationships arethat the toxic response in the individual is causally related to the concentration ofthe chemical, that it depends on the concentration of the chemical at the site ofaction, and that the concentration at the site of action is proportional to the dose.When moving from the individual to a population, allowance has to be made forinter-individual variations in the dose achieving a particular level of response. |
|
|
Quantal/stochastic
Non stochastic
Potency
Threshold effect |
Quantal/stochastic When effect is all or nothing (as with cancer) and it is the minimal dose causing the response that is taken for plotting the dose-reponse relationhip
Non-stochastic When the severity of effect depends on the dose the effect is
Potency The position of the curve provides information on potency of the chemical (the lower the dose at which effects or[for non-stochastic effects] specified levels of effects are seen the greater thepotency). The slope of the curve indicates whether there is a wide concentrationrange over which effects are seen or whether the range is narrow with asignificant increase in toxicity with a relatively small increase in dose
The dose effect curve may also indicate a threshold for the effect. The threshold is a dose level at or below which chemical does not elicit an effect (the no adverse effect level) and above which it does. The concepts of thresholds is critical in risk assessment |
|
|
Toxicodynamics -define |
The process(es) through which a chemical interacts with cells or tissues in thetarget organ, the biological consequences which lead to adverse effects, and thetiming and duration of these interactions and consequences Mode of action The specific way in which a chemical causes toxicity Mechanism of action To describe a much more detailed understanding of the specific way in which toxicity is caused (knowledge of specific biochemical reactions and interactions) The a priori assumption imechanisms of action are common to all mammalian species, and that, if seen inthe usual laboratory species, they also occur in humans. Some mechanisms oftoxicity only occur (or only occur at realistic doses) in certain species; this isknown as species-specific toxicity. A classic example is thalidomide-inducedmalformations in offspring – rabbits and humans are sensitive to this chemical,whereas rats are not. Another example is the light hydrocarbon nephropathy (andassociated renal carcinogenesis) seen only in male rats. Variations intoxicokinetics may also determine relative speciessensitivity. Toxic chemicals interact with and damage cells and biological systems in manyways. These include inhibiting the action of a key enzyme, producing reactive freeradical species, causing direct toxicity to cells (cytotoxicity), stimulating chronic cellproliferation in a tissue (hyperplasia) and binding to receptors on the surface ofcells. |
|
|
Define Toxicodynamics Toxicokinetics |
Toxicodynamics ‘what the chemical does to you and how fastit does it’. Toxicokinetics ‘what you do to the chemical and how fast youdo it’ |
|
|
4 parts disposition of chemical in a body |
Absorption (how the chemical enters the body); Distribution (how the chemical is transported around the body) Metabolism (how the chemical is ‘broken down’ or chemically transformed); Excretion (how the chemical and/or its metabolic products are eliminatedfrom the body). |
|
|
Passage accross the membranes Passive diffusion Facilitated diffusion and active transport Pinocytosis |
Passive diffusion is by far the most common process. It does not require energy.It requires dissolution in the lipid layer of the membrane. Lipid solubility ismeasured by determining the octanol or oil-water partition coefficient, a measurebased on the proportion of the substance dissolved in the octanol/oil relative tothe water. Non-ionised molecules are much more lipid-soluble than ionisedmolecules, and therefore more readily absorbed. Facilitated diffusion and active transport require protein carriers called permeases,and are restricted mainly to chemicals closely resembling compounds normallyfound in the body. Energy is also required for active transport. “Pinocytosis” is a means by which solid particles can be transported by formationof a vacuole (balloon) around the particle for transport across the membrane andrelease on the other side. Passive diffusion requires no expenditure of energy. In both passive andfacilitated diffusion the compound moves down a concentration gradient untilequilibrium is reached. Usually, the flow of air, blood or gut contents past themembrane ensures that a concentration gradient is maintained and equilibriumavoided. Unlike passive diffusion and facilitated diffusion, active transport canmove an agent up a concentration gradient. Some small water-soluble molecules with molecular weights between 100 and 400(e.g. methanol, ethanol, some quaternary ammonium salts) can pass throughaqueous pores in membranes, but the amount absorbed is severely restricted bythe number of pores available. |
|
|
Inhalation |
Considered main route Can be inhaled in forms of gas, vapour, liquid aerosol or solid particulates Gases and vapours - direct exchange between air and blood in pulmonary capillaries, rate depending on liquid solubility. Lipid-soluble compounds are rapidly and almost completely transferred from air toblood; hence their rate of absorption depends on breathing rate. The rate ofuptake of less lipid-soluble materials depends on the air to blood concentrationgradient being maintained, and hence the speed with which blood passes throughthe lung and thus on cardiac output. Thus, uptake is raised in line with work ratewhen manual work is being undertaken. Particles (aerosols, dusts or fibres) may be deposited in the respiratory passagesor the alveoli, or they may be exhaled. Chemicals present in or as liquid (aerosol) particles may be dissolved into fluids atthe site of deposition and hence absorbed. The inhaled substance may also be a solid in the form of dust particles (such ascrystalline silica) or fibres (such as asbestos). These solids may deposit in thelung (although not all particles or fibres deposit in the lung, as is explained later). The ability of particles to penetrate into the respiratory system is determinedmainly by particle size, which is often expressed in terms of the aerodynamicdiameter. This relates to the rate at which the particle settles due to gravity, andis not necessarily the same as the physical diameter of the particle (which may beirregularly shaped). The aerodynamic diameter of a particle is the diameter of aunit density (1g/cm3) sphere that has the same settling velocity due to gravity asthe particle in question. The use of aerodynamic diameter allows for a more directcomparison for particles of different materials and shapes. Particle size is an important toxicological parameter, as it determines where in therespiratory tract the substance can reach and therefore cause toxicity. Within adust or aerosol, there will be a range of particles sizes (the particle-sizedistribution). Ultrafine particles are often deemed synonymous with nano-particles.However, the definitions differ in that, as suggested by the UK HazardousSubstances Advisory Committee and recommended by the European Commission,nanoparticles are defined as particles of size 1-100 nm. |
|
|
Particle size fractions |
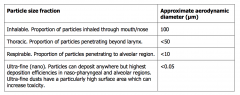
|
|
|
Skin |
Stratum corneum - densely packed keratinised ceels which are biologically inactive, provides main barrier function of the skin Movement of chemicals through stratum corneum is by passive diffusion Extent and rate of p diffusion is determined by - physicochemical properties of the substance (water, lipid solubility, molecular size, degree of ionisation) the solvent the substance is in the surface area of skin covered condition of the skin length of exposure Lipophilic and low molecular weight are well absorbed |
|
|
GI tract |
particulates (dusts) or substances cleared from thelungs by the muco-ciliary escalator can be translocated to the throat andswallowed. The stomach is veryacidic (pH 1-3), whereas the intestine is only slightly so (pH 6). pH can influencethe ionisation of chemicals which can in turn influence the extent of absorption (ingeneral non-ionised forms are more readily absorbed than ionised forms). The intestines contain bacteria, the gut microflora. These may metabolise somesubstances to products which are more readily absorbed and/or more toxic thanthe parent compound. For instance, gut flora can reduce nitrates to nitrites, whichcan cause methaemoglobinaemia, or can react with secondary amines to formcarcinogenic nitrosamines. Substances not absorbed from the GI tract are excreted in the faeces. Substancesmay also be absorbed from the GI tract or from other routes, and be excreted intothe GI tract in bile, for excretion in faeces. |
|
|
Distribution |
Distribution is the movement of the chemical around the body after absorption. occurs in the blood via the circulatory system. Blood circulatesthrough all tissues and there is an equilibration of the level of chemical in theblood and tissues. Some tissues (e.g. liver and kidney) are much more highlyperfused than others and so may receive a proportionally higher dose of thechemical from the bloodstream than other less perfused organs In some cases,chemicals may preferentially accumulate in certain tissues. In particular, lipophilicmaterials (fat soluble) tend to accumulate in body fat (so called adipose tissue);this has been demonstrated with DDT and polychlorinated biphenyls (PCBs). Thisphenomenon is of toxicological significance as it increases the time the chemical isin the body, and there may also be a sudden increase in the concentration of thechemical in the blood when rapid mobilisation of fat occurs, such as in starvationor during diets. Breast milk is lipophilic, so some lipophilic substances canaccumulate in it and be passed on to the infant. The degree of ionisation of the chemical is important as tissues tend to be slightlymore acidic than blood. Weakly basic molecules are ionised to a greater extent inthe slightly more acidic conditions of the tissues and so will pass into the tissuemore readily than they will return to blood. Chemicals may become bound to plasma proteins in the bloodstream, such asalbumin and other plasma proteins. This can serve to reduce the concentration offree chemical in the blood, restrict movement of the chemical into tissues and slowelimination from the body. |
|
|
Biotransfusion (metabolism) |
Absorbed chemicals can be biotransformed (metabolised) by the enzymes that arepresent in the cells of the body. Biotransformation influences both the dispositionand toxicity of the chemical in the body. The liver has the highest metaboliccapacity, with kidney, lung, gastro-intestinal tract and skin also possessingsignificant activity. A chemical may be metabolised at the first site encounteredcapable of metabolism, but as that site often has limited capacity unmetabolisedmaterial is frequently transferred to the liver and metabolised there. If the chemical of interest is a normal chemical encountered in the diet or in air (orresembles such a chemical) it will be metabolised by normal endogenousprocesses. However, most chemicals of interest to occupational health specialistsare foreign compounds (xenobiotics). Most xenobiotics that are absorbed arelipophilic, as this favours absorption. One of the main consequences of biotransformation is to make the chemical morepolar and water soluble, which consequently makes it more readily excretable. Biotransformation can increase or decrease the toxicity of a chemical. If the parentchemical is itself toxic, then rapid biotransformation may reduce the toxicity(detoxification). In contrast, a product of biotransformation may be more toxicthan the parent chemical (metabolic activation or bioactivation), in which casethere may be an increase in toxicity. |
|
|
Excretion |
Excretion (also known as clearance) is the elimination of a chemical from thebody. It is a very important determinant of toxicity. Rapid elimination may reducethe likelihood of toxic effects and their duration. There are several routes bywhich chemicals can be excreted from the body – in exhaled air, urine, faeces,sweat, breast milk and other secretions. In most cases, the most important route of excretion is via the kidneys into theurine. The lungs are an important route of excretion for chemicals, ormetabolites, which are volatile. In this instance, the excretion is by passivediffusion from the blood into the alveolus, down a concentration gradient.Generally, the material exhaled is a low molecular weight gas or vapour. It mayinclude metabolites such as carbon dioxide. Chemicals may appear in faeces either because they have passed through the GItract without being absorbed or because they have been absorbed and excreted inbile. Biliary excretion generally takes place only for relatively high molecularweight material. Excretion via other routes is usually of minor significance. However, as mentionedabove, excretion into the breast milk may be of particular concern as it wouldexpose the suckling child to the toxic chemical. |
|
|
The liver |
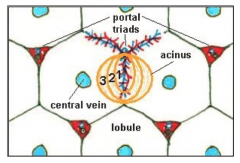
The liver performs a number of important functions - synthesis ofendogenous substances such as proteins, fatty acids, steroids and clotting factor - production of bile and removal of aged erythrocytes (around 80% with remainderremoved by the spleen) -major site of metabolism ofxenobiotics in the body. Hepatocytes (a type of liver cell constituting around 90%of the liver by weight) have a rich range of metabolic enzymes which give them avery high capacity to metabolise xenobiotics. Some chemicals can stimulate thesynthesis of enzymes involved in the metabolism of xenobiotics, in a process calledenzyme induction. This is an adaptive and reversible response to xenobioticexposure, which results in a temporary increase in metabolic capacity. The liver is served by two blood supplies, the portal vein, which supplies around70-80% of hepatic blood, and the hepatic artery, which supplies oxygenatedblood. The portal vein drains the gastrointestinal (GI) tract. Therefore, substancesentering the body from the GI tract first go through the liver before entering systemic circulation. This is the so-called “first pass” and metabolism during firstpass can have a significant effect on the systemic toxicity of a substance, forinstance by extensively detoxifying the chemical before it enters the systemiccirculation. Within the liver these two blood supplies empty together into specialisedcapillaries, the sinusoids. Sinusoids are lined by endothelial cells (which line allblood vessels) and Kupffer cells (resident macrophages of the liver). Thesinusoids are surrounded by hepatocytes and allow the uptake and exchange ofmaterials between the hepatocytes and the blood. The basic functional unit of the liver is considered to be the acinus (from the Latin acinus meaning berry; see Figure 4). The acinus comprises a mass of liver cells and sinusoids centred around the portal triad (containing the terminal branch of the portal vein, hepatic artery and bile duct). Sinusoids radiate out from the triad into the acinus and blood flows from the portal triad towards the central hepatic venules The acinus is subdivided into three zones (1, 2 and 3) on the basis of a gradient in oxygen and nutrient concentration and metabolic activity across the acinus. The zone of hepatic tissue immediately adjacent to the portal tract (zone 1) is rich in oxygenated blood, whilst the peripheral zone 3 around the hepatic veniole is relatively low in oxygen (4-5%). Zone 2 is an arbitrary zone in between zones 1 and 2. It should be noted that classically the functional unit of the liver was regarded as the lobule, a unit arranged around a central hepatic veniole with portal tracts at its periphery. Similarly to the acinus, the lobule was divided into three regions, centrilobular, mid-zonal and periportal regions. Terminology based around the lobule is still widely used to describe regions of pathological lesions in the liver.The level of enzymes and metabolic activity of hepatocytes varies between the three zones of the acinus. For instance, more aerobic metabolism can occur in zone 1 due to the higher concentration of oxygen, and zone 3 hepatocytes have a higher level of cytochrome P450. Consequently, xenobiotics may cause overt toxicity in specific regions, depending on where a toxic metabolite is produced. For instance acrolein causes necrosis of hepatocytes in zone 1, whereas carbontetrachloride causes necrosis in zone 3. Another important function of the liver is the production of bile (a complex mixtureincluding bile acids, bile salts and cholesterol). Bile is released into the intestinesto aid the absorption of lipids. In most species bile is stored and concentrated inthe gall bladder, although some species, notably the rat, do not have a gallbladder . Bile can be an important route of excretion for xenobiotics or their metabolites(biliary excretion). The factors which affect biliary excretion are molecular weight(the threshold molecular weight for drugs and metabolites to be excretedpreferentially into bile varies between species, ranging from ~235 in rats up to500–600 in humans), molecular charge and animal species. Substances excretedin the bile are usually eliminated from the body in the faeces. A potentialconsequence of biliary excretion is entero-hepatic recirculation (EHR). Here, thesubstance excreted in bile comes into contact with the gut microflora (bacteria),which may metabolise it to a form that can be re-absorbed from the intestines andreturned to the liver via the portal venous blood supply. EHR can increase thehalf-life of the substance in the body and exposure of the liver. In some cases(e.g. with 2,4-dinitrotoluene) the gut microflora metabolise the substance to amore toxic chemical, resulting in enhanced systemic toxicity to that of the parentsubstance. Toxicity to the liver is called hepatotoxicity. There are a number of reasons whysubstances can cause hepatotoxicity. It is the first organ exposed to substancesabsorbed from the GI tract, and it will often be exposed to a higher concentrationthan other organs because it is exposed prior to dilution in the systemiccirculation. Also, the liver will metabolise most xenobiotics, and in some cases themetabolites produced may be toxic. These metabolites may cause toxicity in theliver, but may also be released into the systemic circulation to cause toxicityelsewhere. In addition, the liver performs a large number of endogenous metabolic processesand syntheses, and interference with these processes by a xenobiotic can result intoxicity in the liver or elsewhere by virtue of disturbing the homeostasis There are many mechanisms by which substances can cause hepatotoxicity,including inhibiting key enzymes or metabolic processes, interfering with normalfunction, or causing direct cell death. |
|
|
The kidney |
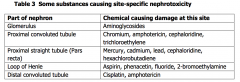
To filter waste products from the blood and toconserve essential substances such as ions (e.g. sodium), water and glucose.Excretion via the kidneys into the urine is a major route of excretion for manyxenobiotics. The functional unit of the kidney is the nephron, which consists of tubules,glomerulus and loop of Henle Excretion into the urine can occur in three ways: - filtration from the blood throughpores in the glomerulus (occurring with small, water soluble molecules), - diffusionfrom blood into tubules - active transport into tubular fluid. Toxicity to the kidney is called nephrotoxicity. Factors include: High perfusion and metabolic rate of the kidney; High delivery rate of toxicants to the kidney; Concentrating role of the tubular structures, especially the proximal tubule; Close proximity of metabolic filtering and absorptive functions. The kidneys have a very high blood flow, receiving around 25% of the cardiacoutput, and thus receiving a proportionally higher exposure than other tissue. Within the tubules, many substances, including water, are re-absorbed, therebypotentially concentrating the xenobiotic in the tubular fluid. This concentratingeffect can be significant with the ratio of the concentration of the xenobiotic in thetubular fluid to blood reaching 500:1 and can lead to toxic concentrations withinthe tubules. For instance, oxalate crystals can form in the tubules after ingestionof high concentrations of ethylene glycol. In a similar way xenobiotics may be re-absorbed with the water and concentrated within the tubular cells. In addition, the kidney has some inherent ability to metabolise xenobiotics, albeitmuch less than the liver. The metabolic activity varies in different regions of thenephron. For instance, the pars recta (proximal straight tubule) has a higherconcentration of P450 than other areas. This can affect where in the nephron asubstance causes toxicity. The environment both inside and outside the nephron varies along its length, aswell as the metabolic capacity, and this influences the type and location of toxiceffects. Table 3 lists the sites in the nephron at which toxicity can occur andchemicals which cause toxicity specifically at those sites. The effects of toxic agents on the kidneys can be assessed easily by theexamination of urine samples which will quickly demonstrate anuria, polyuria,leakage of substances such as glucose, proteins (albumin, globulins) andelectrolytes A battery of renal enzyme function assays is also available inspecialised laboratories. In addition specific markers may be available for certaintoxicities and some markers, such as N-acetyl-β-D-glucosamidase (NAG) are usefulmore generally. There is a characteristic pattern of derangement (excretion ofretinol binding protein, β2-microglobulin or N-acetyl-β-D-glucosamidase) in thecadmium-damaged kidney |
|
|
The respiratory tract |
The respiratory tract includes everything from the nose to the alveoli in the lung.It is clearly a major route of exposure, for substances which can be made airbornecan be inhaled into the respiratory tract. Such substances may cause toxicity inthe respiratory tract itself, or may be absorbed and cause systemic toxicity. The trachea branches to form bronchi, which continue to branch and decrease insize, giving rise to bronchioles. The naso-pharyngeal region moistens the inhaled air and removes particulatematter. Water-soluble or reactive gases (e.g. ammonia and chlorine) are oftenabsorbed in the aqueous secretions of the upper respiratory tract and removedfrom the airstream before it reaches the lung. Nasal tissue can be a target organfor toxicity. Some chemicals, e.g. irritants like sulphur dioxide and formaldehyde,can cause direct tissue damage within the nose. Nasal tissue also possesses somemetabolic activity which can lead to the production of toxic metabolites in situ. Substances may cause toxicity deeper in the lung (pulmonary toxicity),particularly in the alveoli (the gas exchange unit). There are different types of cellwithin the lung which exhibit different levels of metabolic activity and susceptibilityto toxic damage. Clara cells have the highest metabolic activity and are often thetarget for substances that require metabolic activation. Particles deposited in the thoracic region are cleared by the mucociliary escalator(the rhythmic beating of hairs on ciliated cells that line the trachea, moving mucustowards the throat for swallowing). Particles in the alveoli are phagocytosed andcleared by alveolar macrophages. Chemicals can cause toxicity in the lung in a number of ways. Some substances,such as sulphur and nitrogen dioxide, phosgene and ozone can cause directirritation of or damage to lung tissue. This can lead to oedema (leakage of intra-cellular fluid into the lung), which impairs the ability of the lungs to absorb oxygenfrom the air, or cell necrosis. The lung response to damage, characterised byoedema and an influx of phagocytic and other types of cells, is calledinflammation. The lung may attempt to repair damaged tissue by fibrosis, wherefibroblasts release collagen at the site of damage. When caused by exposure todusts, fibrosis in the lung is called pneumoconiosis. Common causes arecrystalline silica (silicosis), asbestos (asbestosis) and coal dust (coal workers’pneumoconiosis). The walls of the lung bronchioles consist of smooth muscle which is under thecontrol of the autonomic nervous system. Signals from nervous tissue to thebronchioles may result in bronchoconstriction or bronchodilation. In asthma thereis inappropriate bronchoconstriction leading to wheezing and difficulty inbreathing. Asthma can be immunologically-mediated where there is an allergicreaction to an inhaled substance (respiratory sensitisation). Isocyanates are classic causes of occupational asthma. Bronchoconstriction mayalso be caused by non-immunological mechanisms. In Reactive AirwaysDysfunction Syndrome (RADS), the airways are particularly sensitive to irritantsand will react if exposed. Lung damage can be detected in a number of ways, including occupationalhistory, structured questionnaire, spirometry, exercise activities and chest imagingtechniques, including X-ray and CT scan. Functional changes may occur, withchanges in respiratory parameters such as forced vital capacity (FVC – the amountof air exhaled by an expiration of maximal effort after a full inspiration) and forcedexpiratory volume (FEV1 – the amount of air exhaled in the first second). Inhumans, some lung conditions such as pneumoconiosis can be detected usingchest X-rays. Immunological tests may be helpful as specific IgE may be formedin occupational asthma and specific IgG formed in extrinsic allergic alveolitis.Obvious damage can be detected using a bronchoscope or histopathologically atpost-mortem. |
|
|
Skin |
The skin comprises the largest organ of the body, with a very large surface area of1.5-2m2 (exceeded only by the surface area of the alveoli). It serves as a barrier,protecting the body from exogenous agents (chemicals, bacteria, fungi etc), maintains internal homeostasis (including temperature regulation and excretion ofsome by-products such as nitrogenous waste products) and provides touchsensation. The skin consists of two main layers; the epidermis and dermis. The outermost layer of the epidermis is the stratum corneum (“horny layer”),which consists of non-living keratinised cells, and provides the main barrierfunction of the skin. The basal layer creates epidermal cells, which graduallymove upwards through the skin becoming increasingly keratinised, eventuallyforming the stratum corneum. The dermis constitutes around 90% of the skinthickness and has a largely supportive function. Subcutaneous fat lies beneath thedermis. This can play an important role in the absorption of lipid solublesubstances. Dermal exposure can cause direct toxicity to the skin and/or systemic toxicity afterabsorption. Chemicals can cause various types of skin damage. One of the mostcommon manifestations of toxicity to the skin is contact dermatitis (inflammationof the skin), which can be irritant or immunological (i.e. a delayed type IV allergicreaction) in nature. Symptoms are erythema (redness), induration (thickening andfirmness), scaling/flaking and vesiculation (blistering) at the site of exposure. Common contact allergens include nickel, glutaraldehyde and epichlorhydrin.When the skin is exposed to reactive substances or substances with an extremepH (i.e. strong acid or alkali) then the skin tissue can be destroyed causing achemical burn (e.g. ammonia, hydrogen peroxide, sodium hydroxide). Chloracneis a potentially disfiguring form of acne, primarily caused by exposure tohalogenated aromatic hydrocarbons. |
|
|
The blood |
The blood is the medium which transports cells and endogenous substances (e.g.hormones, glucose, nutrients) around the body in the vasculature. It consists ofcells – erythrocytes (red blood cells), which carry the oxygen-binding pigmenthaemoglobin, leucocytes (white blood cells), which fight infection, and platelets(which form blood clots) – suspended in plasma. The blood can alsotransport exogenous agents systemically to all tissues in the body, as well as beinga target for toxicity. The haematopoietic system is the organ system thatsynthesises blood cells (bone marrow stem cells). Toxicity towards the blood is called haemotoxicity. There are several ways inwhich toxic chemicals may cause haemotoxicity. One of the most commonmanifestations of haemotoxicity is chemically-induced anaemia, a condition causedwhen the oxygen-carrying ability of the blood is reduced. Anaemia is detectedusing blood counts. There are several mechanisms by which exogenous agentscan cause anaemia: - inhibition of erythropoiesis (production of erythrocytes fromprogenitor cells in the bone marrow), - including inhibition of haem synthesis (e.g.lead), - haemolysis of erythrocytes (haemolytic anaemia), - formation ofmethaemoglobinaemia or binding to haemoglobin to inhibit its oxygen bindingcapability. The bone marrow produces blood cells. It contains stem cells, from which allother cells are derived. Chemicals can cause damage to the bone marrow suchthat the production of a specific cell type or all cell types is reduced(pancytopenia). Chemicals causing pancytopenia include benzene, nitrogenmustards, chloramphenicol and trinitrotoluene. With severe toxicity the ability ofthe bone marrow to produce cells may be completely eliminated, resulting inaplastic anaemia. In haemolytic anaemia, erythrocytes are lysed or damaged, diminishing theirability to transport oxygen around the body and accelerating their removal fromthe circulation by phagocytic cells primarily in the spleen and liver. A variety ofagents can cause haemolysis of erythrocytes, including lead, arsine, and toxins insome animal venoms. In methaemoglobinaemia, the ferrous (Fe2+) iron in haemoglobin is oxidised toferric (Fe3+) iron to form methaemoglobin (MetHb). MetHb binds more stronglythan Hb and therefore prevents effective delivery of oxygen to the tissues. Chemicals causing methaemoglobinaemia include aromatic amines, nitrobenzeneand sodium nitrite. Some chemicals can reduce the ability of haemoglobin to bind oxygen, leading totissue hypoxia. Carbon monoxide binds to the iron in haemoglobin to formcarboxyhaemoglobin, which does not readily carry oxygen. |
|
|
The nervous system |
The central nervous system (CNS), comprising the brain and spinal cord, is whereinformation is sorted, ordered and interpreted, and a range of complex automatic(autonomic) functions are controlled. The peripheral system (PNS) includesnerves which carry activating messages to muscles and sensory messages back(pain, position sense etc). The nervous system, both central and peripheral, is asensitive target for toxic chemicals, and the consequences are calledneurotoxicity. The CNS is protected from toxic chemicals in the blood by the blood-brain barrier(although there are some specific regions not protected by this barrier). Thisconsists of specialised endothelial cells in the brain’s microvasculature, which formso-called tight junctions between themselves to prevent chemicals from passingbetween them to get from the blood into the brain, as occurs with other tissues. Toxic chemicals must instead diffuse through the endothelial cells to enter thebrain – this is largely determined by the chemical’s lipophilicity (solubility in lipids). There are also specialised cells which prevent entry of chemicals into the nervoussystem from adjacent tissues. The brain has a very high demand for glucose and oxygen. Any interference withthe supply or utilisation of these will have adverse effects and possibly causeirreversible cell death due to hypoxia. Hydrogen sulphide and hydrogen cyanide,which inhibit the utilisation of oxygen, and carbon monoxide, which inhibitsoxygen transport, can cause brain damage, secondary to hypoxia. In general, the nervous system has little capacity to regenerate and so damagecan be irreversible. |
|
|
Short term CNS effects and solvents |
Narcosis exposure to high levels of many hydrocarbons including - Propellants (butane) - Cleaning agents (chlorinated solvents) - Aneasthetic agents (halothane) Sudden deaths may occur due to arrhythmias associated with heightened heart sensitivity to endogenous catecholamines At lower but still substantial levels of exposure nervous systemstimulation and euphoria occurs. This is the effect desired from solvent abuse(‘glue sniffing’). Even lower exposure results in drowsiness and impairedreactions, with safety implications when handling dangerous machinery or drivingvehicles. The chemical agents are gases or volatile solvents and generally theeffects are short term |
|
|
Neuropsychiatric disturbances |
Repeated high level solvent exposures may lead to chronic encephalopathies andpermanent neurological effects due to severe and permanent loss of nerve cells. Chronic exposure to low levels of narcotic organic solvents, especially if combinedwith alcohol consumption, may lead to neuropsychiatric disturbances such asdepression, irritability or personality changes. Studies in Scandinavia suggestedthat long term, high level, solvent exposure might be associated with a syndromeof ‘pre-senile dementia’. Some termed this “Danish painter's syndrome” althoughthere was little evidence that the syndrome was so restricted, either by geographyor occupation. Several early studies had methodological flaws and have sincebeen heavily criticised; it seems likely that some studies had significant biases andearly attempts to reproduce these findings out with Scandinavia wereunsuccessful, casting doubt on the existence of this syndrome. ‘Grand mal’ type epileptic seizures have been ascribed to exposure to high levelsof methyl bromide, an ozone depleter, formerly used as a steriliser for soil and for containers carrying imported crops such as bananas. Use of this substance is nowbanned under the Montreal Protocol. Mood disorders, personality changes, impaired intellectual function, or sleepdisturbances with vivid dreams or insomnia may occur following exposure tomercury (‘mad as a hatter’ following exposure to mercuric chloride during feltingof hat materials), and carbon disulphide. Chronic exposure to manganese cancause an acute organic psychosis (manganese madness), with Parkinson’s disease-like symptoms in more severe cases. |
|
|
Organophosphate induced delayed neuropathy |
Many organophosphates are in widespread use as pesticides and veterinarymedicines. Others are potential chemical warfare agents. The two main effectsare acute toxicity due to anticholinesterase activity and organophosphate induceddelayed neuropathy (OPIDN), which results from inhibition of neurotoxic esteraseactivity in the nervous tissue. Carbamate pesticides also show this type of effect. Early signs of anti-cholinesterase poisoning include headache, nausea, anorexiaand lassitude, as well as constriction of the pupils. Vomiting, diarrhoea, abdominalpain, muscle twitching and incontinence lead to convulsions, coma and possiblydeath. The patient should be treated with atropine, and supported by mechanicalrespiration. Pralidoxime (PAM, P2S) is administered to restore cholinesteraseactivity. Blood cholinesterase activity can be used as a biochemical marker forthe poisoning. Pralidoxime should not be used for carbamate poisoning. OPIDN tends to be most visible as paralysis of the legs, and occurs 7-14 days afterexposure. There is no specific treatment for this effect, although some recoveryusually occurs. The organophosphorus agent binds to the neuropathy targetesterase (neurotoxic esterase) and inhibits it. A predictive animal test using hens used to be used to identify compounds capableof OPIDN. After administering the organophosphorus compound (with atropine tosuppress the anticholinesterase effects if necessary) the OPIDN effect wasfollowed by examining the hens’ gait. This has now been largely superseded bystudies on the binding of organophosphorus compounds to neurotoxic esterase invitro. Claims that neuropsychiatric disturbances may occur in sheep dippers and GulfWar veterans following exposure to organophosphates are still under investigation. |
|
|
Hexacarbon induced peripheral sensorimotor neuropathy |
Hexacarbon neuropathy (a sensorimotor peripheral neuropathy) has beenidentified in studies on Italian shoe workers, Taiwanese press proofers andworkers in a factory producing tungsten carbide alloys. A ‘glove and stocking’distribution of sensory impairment (to touch, pain, vibration) is sometimes used todescribe the sensory neuropathy. The accompanying motor neuropathy canpresent as apparent weakness and atrophy of limb muscles proximal to theextremities. Hind limb weakness, leading to paralysis, is observed in animalstudies. Nerve conduction velocity is significantly impaired. This is associatedwith swelling of the distal portion of the nerve axons, dieback of the myelinsheath, and axonal degeneration and loss. n-Hexane, methyl-n-butyl ketone and 2,5-hexanedione (all of which share a common metabolic pathway leading to aninteraction with lysine molecules in proteins) are capable of causing theneuropathy; methyl-ethyl ketone and acetone appear to potentiate the effects,probably because of their influences on this common metabolic pathway |
|
|
Other effects |
developmental effects due to exposure to leadand organic solvents, including exposure during pregnancy. There are specificregulations controlling workplace exposure to lead (Control of Lead at WorkRegulations, 2002) |
|
|
Neuropsychogenic disease (somatogenic disease) |
Mass psychogenic illnessand idiopathic environmental intolerance. Mass psychogenic illness is ’the rapidspread of illness signs and symptoms for which there is no plausible organicaetiology. Episodes are typified by an anxiety generating precipitant within thevictims’ immediate environment, and symptoms occur within close temporalproximity of exposure to the stimulus. Idiopathicenvironmental intolerance is ‘a subjective illness in certain persons who typicallydescribe multiple symptoms, which they attribute to numerous and variedenvironmental chemical exposures, in the absence of objective diagnostic physicalfindings or laboratory test abnormalities that define an illness’. Often the diagnosis for these illnesses is by exclusion. Although the illnesses maybe ascribed (by those affected) to workplace exposures, and therefore appear toinvolve chemical toxicology, they are essentially psychological in origin and needto be treated as such. They are included here because it is necessary, wheninvestigating incidents or cases, to be aware that sometimes scepticism is requiredwhen dealing with claims of chemical causation. |
|
|
The immune system Cell mediated immunity Humeral immunity |
Cell mediated - lymphocytes that are sensitised for a specific antigen Humoral immunity - involves production of immunoglobulins (antibodies) Chemicals can interfere with the normal functioning of the immune system,termed immunotoxicity. They can do this by having an adverse effect in tissuesthat are involved in the production or functional maturation of cells of the immunesystem, such as bone marrow and thymus, or interfere with some aspect of thecomplex chain of events involved in immune function. Chemicals can causeimmunosuppression, where the effectiveness of the immune system is reduced.Examples of chemicals causing immune suppression are polybrominated biphenyls(PBBs), organophosphorus and organotin compounds, and drugs such ascyclosporin. More commonly, exogenous agents may stimulate an exaggerated immuneresponse towards themselves, called sensitisation (hypersensitivity). Sensitisationrequires two phases, induction and elicitation. To elicit an immune response,chemicals must be large enough to be recognised by the immune system as anantigen. Large molecules (>3000-5000 daltons) may be recognised by theimmune system. However, small chemicals are too small to act as antigens, andmust bind to endogenous proteins or other macromolecules to form a largerconjugate than can stimulate an immune response (i.e. they act as haptens). Firstexposure leads to a sensitised state where the immune system is primed torespond to the antigen. Subsequent exposure (challenge) leads to elicitation,where an overt immune response occurs. Sensitisation reactions in response tochemicals usually occur in the skin (allergic dermatitis) and respiratory tract(asthma). Common causes of skin sensitisation are nickel, formaldehyde andepoxy resins. Isocyanates and wood and flour dust are well known causes ofoccupational asthma. |
|
|
Other organs |
The eye can be exposed to chemicals directly from the air, and also from thesystemic circulation. There are several sites within the eye that can be damagedby toxic agents, including the lens (forming cataracts), cornea and retina/opticnerve. Examples of ocular toxins are methanol (damages optic nerve causingblindness), and 2,4-dinitrophenol and naphthalene which can cause cataracts. The ear contains both the end organs of hearing and of balance. Hearing loss isthe common effect of ototoxins. Many agents also affect the vestibular (balance)system, but there are few agents known to be exclusively vestibulotoxic. In theear, the hair cells within the inner ear (which translate sound into nerve impulses)and the vestibulocochlear nerve (which sends the nerve impulses to the brain) aretargets for toxicity. Many drugs and chemicals are considered to cause toxicity tothe ear (ototoxicity). Aminoglycoside antibiotics such as gentamicin and neomycinare a well-known example of ototoxins, causing both toxicities. Other chemicals,including cisplatin, some types of diuretics, styrene and trichloroethylene have alsobeen implicated in causing hearing loss. |
|
|
Reproductive toxicity |
Four phases to the reproductive cycle, which cover the stages ofpre- and post-natal development (pre- and post-birth), sexual maturation andmating. Chemicals can cause adverse effects in any of these phases, collectively known as reproductive toxicity. Reproductive toxicity canbe divided into two categories, adverse effects on reproductive capacity (fertility)and adverse effects on the developing foetus (developmental toxicity). Impaired fertility (ability to achieve the normal number of established pregnanciesand viable offspring) can result from toxicity to any stage of the reproductive cycleup to and including the implantation of a fertilised ovum in the uterus. A chemical may cause reduced fertility through altered libido or through effects ongametogenesis (production of gametes, i.e. sperm or ova). Altered libido is almostimpossible to demonstrate in the individual. Spermatogenesis (production of sperm) in the testes is a complex process wherespermatogonia (primitive germ cells) mature and differentiate into spermatozoa(mature sperm) through a number of stages (formation of spermatocytes [which undergo meiosis, to halve the number of chromosomes present], spermatids, andsperm). Millions of sperm are produced daily from puberty onwards. Exposure tochemicals that interfere with spermatogenesis or with the quality of spermproduced can reduce male fertility. Given the continuous production of sperm,adverse effects of a chemical on spermatogenesis or on the sperm themselvesmay be reversible upon cessation of exposure, provided that the spermatogonia ortestes are not irreversibly destroyed. Autogenesis is the production of the female gametes (oocytes) in the ovaries. Incontrast to sperm, which are produced constantly through adult life, femalegametes are produced in the ovaries and no more are produced after birth. Afterbirth a high proportion of oocytes are destroyed naturally through atresia, and asmall number mature into ova (“eggs”) and are released during ovulation.Oocytes are potentially vulnerable to toxic chemicals as they cannot be replacedand they could accumulate genetic damage which could be passed on to theoffspring if the ovum is fertilised. A high profile contemporary issue is that of endocrine disruptors. These arechemicals which interfere with the normal hormone balance and function in thebody. Given that most aspects of reproductive function are controlled byhormones, endocrine disruptors can cause reproductive toxicity, manifested inways such as reduced sperm number and quality, and cause menstrualdisturbances. Developmental toxicity is an adverse effect on the normal development andgrowth of the offspring, through the foetal stage to attainment of sexual maturity.It is manifested as death of the foetus, structural abnormalities (e.g. cleft palate,shortened limbs), altered growth or functional deficiency. The induction ofstructural abnormalities is often called teratogenesis (from the Greek for “monsterforming”). These abnormalities can occur externally or in the skeleton or softtissue (visceral), and are generally categorised as malformations (permanentstructural change outside the normal biological range that have a serious adverseeffect on the foetus and may impact on survival, e.g. spina bifida, cleft palate,heart defects) or variations (a change that occurs within the normal populationand is unlikely to have a significant impact on survival or health, e.g. wavy rib).Malformations are more toxicologically significant than variations. Specific organs and tissues develop and mature at different times duringgestation. As a result, the time at which exposure to a teratogen occurs candetermine which particular organ or structure is vulnerable to damage. Forinstance, exposure during the first trimester, when the organs are developing,might cause a malformation in a specific organ. Exposure to the same chemical inthe last trimester may not cause the effect as by this stage the target organ isalready formed. Endocrine disruption caused by chemicals is becoming a notable issue. Onedefinition of an endocrine disruptor (WHO/UNEP 2012) is: “An endocrine disruptor is an exogenous substance or mixture that altersfunction(s) of the endocrine system and consequently causes adversehealth effects in an intact organism, or its progeny, or (sub) populations”. Endocrine disrupting chemicals may: - Mimic or partly mimic naturally occurring hormones like oestrogens,androgens and thyroid hormones; - Bind to hormonal receptors (anti-oestrogens and anti-androgens); - Interfere or block the way natural hormones or their receptors are made or controlled. They are also claimed to cause feminisation in male offspring. These chemicals include a number of phthalate esters (notably diethylhexylphthalate and dibutyl phthalate), and other plasticisers (such as bisphenol A)used in the manufacture of a variety of films used for wrapping food.Phytoestrogens (naturally occurring substances in plants that have hormone-likeactivity) such as genistein (from soy containing products) may also act asendocrine disruptors. The contraceptive pill is an intentional oestrogen disruptingchemical used therapeutically to impair fertility, so when it is used therapeuticallythe impaired fertility is not an adverse effect. Should workplace exposure result inimpaired fertility, this could be considered an adverse effect and the contraceptivepill an endocrine disruptor. How to assess and react to the presence of endocrine disrupting chemicals is stillan area of controversy. Only recently have suites of appropriate tests andsuggestions as to criteria for judging the risks become available, although theyare not yet universally accepted. |
|
|
Genotoxicity |
The genetic material of a cell (the genome) is contained within its nucleus in theform of deoxyribonucleic acid (DNA). In humans and animals the genome ispackaged into discrete chromosomes (23 pairs in humans). Genotoxicity is a general term to describe adverse effects on the genetic materialcaused by chemicals. More specifically, mutagenicity refers to the induction ofpermanent heritable changes in the amount or structure of the genetic material ina cell. There are several types of mutagenic activity. Mutagenic changes range from changes in one or a few DNA base-pairs (gene mutation) to gross changes inchromosome structure (chromosome aberration) and changes in the number ofchromosomes: aneuploidy (an abnormality involving a chromosome number that isnot an exact multiple of the haploid number [one chromosome set is incomplete])and polyploidy (having one or more extra sets of chromosomes). Chemicalscausing chromosome aberrations are called clastogens, and those causinganeuploidy, aneugens. Mutagenic changes can occur in somatic cells or germ cells. Somatic cellmutagens are usually carcinogens and so the main concern for mutations insomatic cells is the subsequent development of cancer. Mutations in germ cellsmay lead to the transmission of genetic damage to subsequent generations, whichcould manifest as cancer or other genetic disease in the offspring. To date, allknown germ cell mutagens are also somatic cell mutagens. A large number of tests have now been developed for predicting genetic toxicityand most have undergone validation. Some of these tests are widely acceptedand now in regular use. The current convention concerning these assays is toconcentrate on in vitro assays, and only to perform in vivo assays when there is aneed to do so following positive in vitro results. There are now four in vitro assays regularly used, and a battery of three, includingthe bacterial reverse mutation assay, is usually regarded as sufficient fordemonstrating a negative. The usual choice for the first two assays is thebacterial reverse mutation assay and either the chromosomal aberrations assay orthe more recently approved in vitro UDS (unscheduled DNA synthesis) assay.Normally the preferred third assay is the assay for point mutations in mammaliancells. |
|
|
Bacterial reverse mutation assay |
The Ames test (the original and still the most used) employs strains of Salmonellatyphimurium which require histidine for growth and which have an increasedsensitivity to mutation by virtue of a decreased repair capacity and morepermeable cell wall. Under conditions of low histidine concentration, only bacteriawhich have reverted to histidine independence are able to grow into visiblecolonies. A similar system, using tryptophan-dependent Escherichia coli, is alsowidely used. In both cases a metabolising system (from induced rat liver) is alsoincorporated to encourage detection of active metabolites |
|
|
Chromosomal aberration test in vitro |
In this test cells (typically human lymphocytes or Chinese hamster solid tissuecells) are incubated with the chemical. The cells are then allowed to divide beforeinserting an agent that blocks cell division at the metaphase stage, and the cellsare stained to reveal the chromosomes Chromosomal and chromatid breaks andanomalies are examined following incubation both with and without a metabolisingsystem. |
|
|
Point mutations in mammalian cells |
The assay uses cultured mammalian cells. Essentially the aim is to examine if cellcolonies are formed after treatment with the chemical and growth in theappropriate medium deficient in a key substance (e.g. thymidine when a TK+[thymidine kinase containing] mutation is being sought). Again the test isperformed with and without metabolic activation. |
|
|
In vitro UDS (unscheduled DNA synthesis) assay |
In essence this assay looks for increased DNA synthesis following treatment ofrodent liver cells with chemical. It is based on the requirement to excise and repairthe DNA. DNA synthesis is measured by autoradiography or by liquid scintillationcounting after growing the cells in the presence of radioactive (tritiated)thymidine. |
|
|
In vivo assays |
In vivo assays are also available, but are now only used as supplementary tests.They include the micronucleus assay (mouse) and in vivo bone marrowcytogenetics, in vivo/in vitro UDS and mouse spot test. Older assays in yeast andfruit fly (Drosophila) may still be valuable when interpreting genotoxicity. |
|
|
Carcinogenicity |
This difficult subject area has been central in occupational toxicology for the lastthree or four decades. Increasing understanding of the biology of carcinogenesishas been combined with an expanding programme of epidemiological studies inworker populations to stimulate and direct toxicological research. It is necessaryto understand that these disparate branches of science feed off each otherinteractively quite intensively so that, whilst it is convenient to learn the disciplinesof each subject separately, they are in real life practice often inextricablyintertwined. Thus, for instance, an epidemiology study of oil workers may identify, as a novelfinding, a specific cancer, for example haemopoietic, associated with exposure to aspecific agent. If the effect is large or the chemical is important or widely usedthen its action is likely to be explored in animal studies to ascertain dose-responserelationships, metabolic activation, etc. Similarly, in biology, the identification ofspecific oncogenes (cancer causing genes) implicated in haemopoiesis may lead toinvestigation of how they might be activated by specific chemical agents or theirmetabolites. |
|
|
Biology of cancer |
The multistage model incorporates events which may be genetic or non-genetic(epigenetic) at initiation, although most industrial chemical carcinogens exert theireffect at this stage genetically It is reckoned that in humans there are 8 - 10cancer-initiating events per day. However it will be seen from the figure that ateach stage of carcinogenesis there is a tendency to curtail the carcinogenicprocess and to return to a homeostatic state. Apoptosis is a principal contributorto this return to homeostasis. Defective apoptosis represents a major factor in thedevelopment and progression of cancer. There are two main pathways for theinitiation of apoptosis, mitochondrial regulation (release of proteins activatingcaspases) and direct signal transduction involving the tumour necrosis factor(TNF) receptor, both of which involve extensive cellular signalling and lead torelease and activation of caspases. Activated caspases degrade intracellularproteins, which leads to cell shrinkage (pyknosis), with dense and tightly packedorganelles, chromatin condensation, karyorrhexis (DNA fragmentation) anddevelopment of several discrete chromatin bodies, blebbing of the cell membraneand break-up of the cell to form apoptotic bodies, which are phagocytosed. Whenthe cell signalling breaks down this can lead to malfunction of the apoptoticpathway and continuing cell growth and division, leading to tumour formation. Itfollows that cancer when it manifests overtly may be seen as the (relatively) raresurvivor of multiple surveillance processes which have been evaded. As well as accommodating possible biological mechanisms the model also helps tounderstand the concept of latency, the time-space between an occupationalexposure (to an initiator or promoter) and the consequent cancer Latency for occupational agents varies from 2 years for leukaemia caused by radiation to 10years for solid tumours caused by radiation. β-Naphthylamine or benzidineexposure may result in bladder cancer 8 - 20 years later. Lung cancer andmalignant mesothelioma from mineral fibres, silicates and crystalline silica may notoccur for 30 - 60 years after exposure. |
|
|
Carcinogenicity testing - in vivo methods |
Mutagenicity testing has an imperfect concordance with the in vivotests which we are going to consider in this section. Biologically this observation isunsurprising since it defies reason to believe that any simple, isolated, in vivo or invitro test is likely to mimic the subtle interplay of carcinogenic influences in thewhole animal. The use of short term tests (including mutagenicity assays and cell transformationassays) thus raises a set of concerns and problems which are both scientific(interpretative) and regulatory. The tests themselves have intrinsic value inhelping to determine how a particular substance is to be viewed as a carcinogenichazard, and will also inform decision making on the design of further studies inanimal models. The gold standard of in vivo testing remains the lifespan animal study. In thismethodology, usually carried out in rodents, a population of animals is dosed witha specific agent for some or all of their lifespan. The animals are observed andexamined pathologically according to a pre-determined regime. The underlyingrationale of this approach is that it mimics, in a naturalistic way, the experience ofhuman populations and permits the expression of the full range of potentialcarcinogenic effects that such a population might experience. The difference oflifespan between man and animals cannot, unfortunately, be overcomecompletely. Such classical studies have been pursued for several decades and there is thusconsiderable experience of them with regard to their predictive value for humanpopulations. Because of experience with species differences (and metabolicactivation) the full gold standard test uses two species (normally rat and mouse)rather than one. The route of exposure is important. For occupational exposureinhalation is the preferred route but the difficulties associated with operatingexposure chambers often means that oral exposure (by dietary administration) isused. Positive results from studies using parenteral administration are oftenregarded as being of limited value. This traditional approach is very time-consuming and very costly and its use isincreasingly confined to pharmaceutical and pesticide development. Shorter termin vivo tests (often employing genetically modified rodents) have a role filling thegap between these classical studies and the batteries of in vitro tests and in vivomutagenicity assays. |
|
|
CHIP4 and the european regulation on classification, labelling and packagine substances and mixtures |
The purpose of classification is to formally highlight the hazards of a chemical (ormixtures containing hazardous chemicals) - to ensure that people who aresupplied with chemicals receive the information they need to protect themselves,others and the environment. In UK law classification and labelling is currently implemented through theChemicals [Hazards Information and Packaging for Supply] Regulations (newedition – CHIP4, 2009) and through EU Regulation 1272/2008 (the classification,labelling and packaging [CLP] Regulation). This is an interim measure. Both setsof legislation require that a substance or mixture has to be classified in accordancewith certain rules that were/are common through the EU. If the substance isfound to be ‘dangerous’, the label must contain standardised informationconcerning hazard and the substance/preparation must be packaged suitably.There must also be a label on the container which lists the classification in theform of symbols (e.g. skull and crossbones) and standard phrases. The EU CLPRegulation is superseding the UK CHIP Regulations and introducing the UN‘Globally Harmonised System’ (GHS) of phrases and labels into the EU as a whole,including the United Kingdom. Substances already have to be labelled inaccordance with the new system; mixtures may still be labelled under the oldsystem until June 2015. Separate legislation (the EU REACH Regulation, EU Regulation 1907/2006)requires that chemicals and preparations must be supplied with a safety datasheet that outlines the hazards of the product and how it can be handled safely. The classification criteria cover all toxicological endpoints (includingcarcinogenicity, mutagenicity, reproductive toxicity, skin and eye irritation, skincorrosivity, skin and respiratory sensitisation, repeated-dose toxicity and acutetoxicity). |
|
|
COSHH and WELs |
The Control of Substances Hazardous to Health Regulations (2002)(COSHH), as amended, require employers to conduct an assessment of the risksto workers through exposure to ‘substances’ (chemical and biological agents). Itis based on the Chemical Agents Directive (EU Directive 98/24/EC). Exposures tolead, asbestos and physical agents are controlled separately. Toxicity information,usually derived from the materials safety data sheet or the trade literature, isrequired when undertaking these assessments. Toxicity information is also usedby the EU and HSE to set workplace exposure limits under COSHH. Theseexposure limits are listed in EH40 (check the HSE website for the latest version)published by HSE Books. In house limits may be required for those chemicals notincluded in EH40. |
|
|
REACH |
REACH (EU Regulation 1907/2006) is a relatively new European Union regulationconcerning the Registration, Evaluation, Authorisation and restriction ofCHemicals. It came into force on 1st June 2007 and replaces a number ofEuropean Directives and Regulations with a single system. This Regulation requires the documentation of certain toxicological information onall chemicals and hence makes open the basis for the classification of a chemical. It includes the provision of materials safety data sheets and, for higher tonnagechemicals, chemicals safety reports. Further, if certain types of dangerousproperty are identified, ‘Authorisation’; will be required, and will only be grantedfor specified uses of the substance. ‘Authorisation’ will not be permitted if suitablesubstitutes are available. The implementation of REACH is taking place over a‘phase-in’ period. Registration of new substances has already been implementedExisting substances are being registered in groups, starting with those substancesof high tonnage (1000 tonne/y) and those substances with known specifieddangerous properties (CMR – carcinogenic, mutagenic, toxic to reproduction,categories 1 or 2 and 1 tonne/y or ‘may cause long term adverse effects on theaquatic environment’ and 100 tonne/y) in 2010, and finishing with those placed onthe market in quantities of 1 tonne/y in 2018. |

