![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
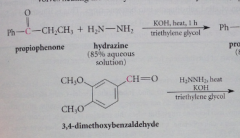
Wolff-Kishner rxn: typically uses ethylene glycol or similar high-boiling cmpds as co-solvents because
|
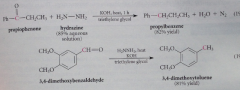
high bp of solvents allow rxn mix to reach high temp required for reduction at reasonable rate
|
|
|
Hydrazone
|
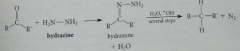
Wolff-Kishner intermediate followed by Bronsted AB rxns => expulsion of dinitrogen gas & formation of product
|
|
|
The Wolff-Kishner reduction takes place under strongly ___ conditions
|
basic
|
|
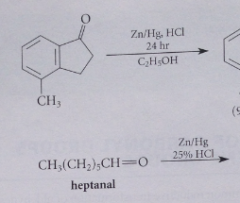
Clemmensen reduction
|
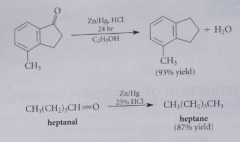
Aldehyde or ketone is reduced under acidic conditions w zinc amalgam (solution of zinc metal in mercury) in presence of HCl
|
|
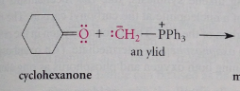
Wittig alkene synthesis
|
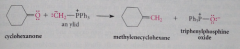
Addition-elimination preparation of alkene from aldehyde/ketone
|
|
|
The Wittig synth. is important bc it gives alkenes in which
|
position of db is regioselective
|
|
|
ylid
|

any cmpd w opposite charges on adjacent, covalently bound atoms, each of which has an electronic octet
|
|
|
Resonance structures for phosphorus where one is uncharged
|
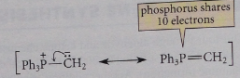
|
|
|
Wittig alkene synthesis mechanism
|

The anionic oxygen reacts w phosphorus to form an oxaphosphetane intermediate (4-membered ring w O & P) which undergoes B elimination to give alkene & triphenylphosphine oxide
|
|
|
Wittig mech 2
|
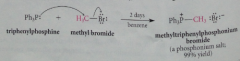
The ylid starting material prepared by rxn of an alkyl halide w Ph3P in an Sn2 rxn to give a phosphonium salt
|
|
|
Wittin mech 3
|
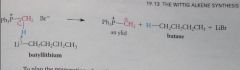
phosphonium salt converted into its conjugate base, the ylid, by rxn w a strong base such as an organolithium reagent
|
|
|
Wittin syntheses are planned so that the most ___ alkyl halide can be used as a starting material
|
reactive: since the rxn to form salt is SN2, fastest w methyl & primary alkyl halides
|
|
|
Problem with wittig alkene synthesis
|
mixture of e and z isomers
|
|
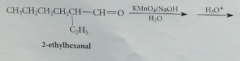
|
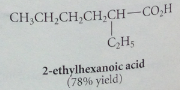
|
|
|
Some aldehyde oxidations begin as addition rxns such as oxidation of aldehydes by Cr(VI) reagents, where the hydrate, not the aldehyde, is the species oxidized
|
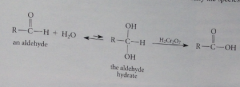
The alcohol is the hydrate formed by addition of H2O to the aldehyde carbonyl group, so some H2O should be present in solution so that aldehyde oxidations with Cr(VI) occur at a reasonable rate
|
|
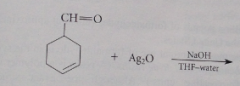
|
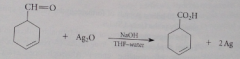
Handy when aldehyde has db or alcohol OH groups which react w oxidizing reagents but not Ag2O
|
|
|
Tollens test
|
If the silver ion is solubilized as its ammonia complex +Ag(NH3)2, oxidation of the aldehyde is accompanied by the deposition of a metallic silver mirror on the walls of the rxn vessel
|
|
|
Many aldehydes are oxidized by ___ by standing for a long time
|
O in air
|
|
|
Can ketones be oxidized w/o breaking C-C bonds?
|
No
|
|
|
Ketones are resistant to ___ but oxidized by ___
|
Cr(VI) reagents (even acetone can be used as a solvent) --- potassium permanganate (not useful as oxidizing reagent)
|
|
|
Manufacture of formaldehyde
|

oxidation of methanol over a silver catalyst
|
|
|
phenol-formaldehyde resins
|

polymer w rigid 3D network of repeating units
|
|
|
How are phenol-formaldehyde resins produced?
|
Variation of FC alkylation inw hich phenol & formaldehyde heated w A/B catalysts
|

