![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
41 Cards in this Set
- Front
- Back
|
Nucleoside vs Nucleotide? (P.353)
|
Nucleoside is a glycosidic cmpd attached to a pentose sugar.
Nucleotide is an ester cmpd with a sugar and phosphate group attached. |
|
|
Re Purines and Pyrimidines, at what positions do they differ? (P.353)
|
Purines differ only at positions 2 and 6.
Pyrimidines differ at positions 4 and 5. |
|
|
What is the precursor to all of the purines? Pyrimidines? (P.353)
|
Purines = Hypoxanthine
Pyrimidines = Uracil |
|
|
In purines, where is the pentose typically attached? Pyrimidines? (P.354)
|
Purines = C9
Pyrimidines = C1 |
|
|
Where is the phosphate group typically added in the pentose sugar?
|
C5
|
|
|
Three sites of modification occur in pentose sugar. What are the locations and what happens there?
|
C1 = glycosidic linage
C2 = oxy or deoxy occurs here C5 = Phosphates |
|
|
Phosphates can be labelled alpha, beta, or gamma - which one is alpha?
|
Alpha = closest to pentose ring.
|
|
|
Cancer treatments often target this pathway: (P.355)
|
Nucleotide metabolism
|
|
|
What is the starting compound in purine de novo synthesis? What is the final compound before a branch point is reached? (P.355)
|
PRPP = starting material
IMP = final cmpd before branching occurs |
|
|
What 4 aa's are used in purine nt synthesis and what doe they contribute? (P.355)
|
Glutamine (1N), Glycine (1N, 2C), Glutamine (1N) and Aspartate (1N)
|
|
|
how many ATP are required to make purine? (P.355)
|
5 (4 for energy plus the original precursor molecule PRPP)
|
|
|
How many carbon and nitrogens does glycine contribute? (P.355)
|
2Cs and 1 N
|
|
|
What does chemo drugs typically target in purine pathway? How many "formylations" does the purine synthesis rxn use and what doe they contribute?? (P.355)
|
Tetrahydrofolate - uses two THF, each contributing 2 Cs
|
|
|
How many CO2 molecules does the purine synth pathway use and how many Cs does it contribute?
|
1 CO2 and 1 C
|
|
|
When purine synthesis reaches IMP, the pathway branches - what do the two branches make?
|
AMP and GMP
|
|
|
What aa does the AMP branch use? How/where is IMP modified? What is its energy requirement? (P.356)
|
AMP uses Aspartate; Oxygen at position 6 of IMP is replaced with amino group; Requires GTP
|
|
|
What aa does the GMP branch use? How/where is IMP modified? What is its energy requirement? (P.356)
|
GMP uses Glutamine; Amino group is added at IMP position 2. Energy requirement is ATP
|
|
|
Where does the D-ribose-5-phosphate precursor to PRPP come from?
|
Pentose phosphate pathway
|
|
|
D-ribose-5-phosphate to PRPP via PRPP synthetase is NOT the committed step on de novo purine synthesis. Why? (p. 356)
|
Bc PRPP can be used in other pathways and is not strictly used for purine nt synthesis.
|
|
|
Overexpression of PRPP synthetase or increased enzymatic activity with altered kinetic properties has been documented in small subsets of what population?
|
Gouty pts
|
|
|
What enzyme is involved in the committed step on purine synthesis? What does it do? (p 357)
|
ATase (Amidophosphoribosyltransferase). It desplaces pyrophosphate with the amino group of glutamine.
|
|
|
How is ATase regulated (p 357)
|
It is a dimer with its active site hidden. When PRPP is high enough, it induces monomerization and allows catalytic activity. When it purine nts get high enough, ATase is induced to dimerization/inactivation.
|
|
|
What are the three multifunctional enzymes that are used in purine synthesis? Why does nature use these?
|
GART, AIRC and Adenylosuccinate lyase. These enzymes are potentially used for three reasons:
1. Unstable intermediates 2. Efficiency 3. Toxic intermediates |
|
|
Synthesis and interconverstion of nucleoside diphosphates and triphosphates are mediated by different kinases. Which are base-specific and which are broadly specific? (p358)
|
Monphosphate kinases are base-specific (Eg Adenylate Kinase and Guanylate Kinase). Diphophate kinases are broadly specific.
|
|
|
Adenylate cyclase is important bc it regulates the ratio of ATP, ADP and AMP to what? (p358)
|
100:10:1, respectively
|
|
|
Review figure on p 359 for says that purine synth is regulated
|
Review figure on p 359 for says that purine synth is regulated
|
|
|
What are the three key rxn in catabolysis of purine nts?
|
1. Dephosphorylation
2. Deamination 3. Phosphorolysis |
|
|
In the first step of AMP degradation, two paths can be taken - this results in ?? or ?? Why is this important? (p359)
|
IMP or Adenosine. IMP is produced in exercising skeletal muscle and facilitates the net resynthesis of ATP following exercise. Adenosine is produced in ischemic or anoxic heart - it is a potent coronary vasodilator that facilitates oxygen delivery back to damaged tissue.
|
|
|
Memorize basic degradation pathway on pg 359?
|
Memorize basic degradation pathway on pg 359?
|
|
|
How does ischemia cause increase production of free radicals?
|
XDH and XO are used to convert hypoxanthine to uric acid. Under ischemic conditions, XDH is converted to XO and XO uses O2 to produce Xanthine and hydrogen peroxide as a byproduct. The H2O2 is toxic
|
|
|
What are the three ways someone can have Primary Gout? (p361)
|
1. Overexpression of PRPP
2. Deficiency of HPRT salvage pathway enzyme 3. Urate excretion deficiency |
|
|
What two things can cause Secondary Gout?
|
1. Drug intake
2. Unusual dietary habit |
|
|
What is allopurinol and what is the mechanism by which it works? (p361)
|
It is an analogue of hypoxanthine that inhibits XDH (xanthine dehydrogenase). This shunts excess hypoxanthine through the salvage pathway, increases nts that inhibit ATase and therefore urate.
|
|
|
Second to Urate, what is the most insoluble purine base in the body? What deficiency causes this? How is accumulation of this compounded treated?
|
Xanthine. Excess caused by a deficiency in Xanthine Dehydrogenase. Only way to reduce this is via diet.
|
|
|
What two deficiencies cause SCID?
|
Adenosine Deaminase (ADA) and Purine nucleoside phosphorylase (PNP).
|
|
|
What is the mechanism by which ADA and PNP cause SCID?
|
dATP or dGTP accumulates in lymphocytes. This excess inhibits ribonucleotide reductase responsible for producing all of the dNTPs necessary for DNA synthesis.
|
|
|
What are the two ways that purine nucleotides can be produced in the salvage pathway? How much energy is required? (p363)
|
1. a) Adenine phosphoribosyltransferase (APRT) attaches PRPP directly to adenine to produce AMP
b) Hypoxanthine-guanine phosphoribosyltransferase (HPRT) can add PRPP directly to Hypoxanthine or Guanine to produce IMP or GMP, respectively. 2. ATP can be directly attached to a purine nucleoside. **Both require 1 ATP vs 5 |
|
|
In Lesch-Nyhan syndrome, what pathway is defective and what enzyme is deficient? (p364)
|
Salvage pathway is defective and caused by deficiency in HPRT
|
|
|
What is the inheritance of Lesch-Nyhand syndrome? (p364)
|
X-linked
|
|
|
With a deficiency in HRPT, what symptoms would you expect? (p364)
|
You would expect a decrease in salvage pathway shunting and an increase in Urate and therefore gout. There would also be an increased rate of PRPP synthesis and de novo purine synthesis.
|
|
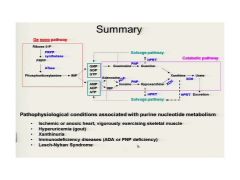
A nice review of lecture 1
|
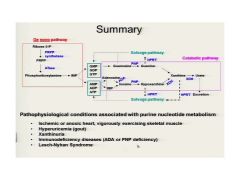
A nice review of lecture 1
|

