![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
Blank
|
Blank
|
|
|
Describe the structure and function of the nuclear envelope
|
The nucleolus (also called nucleole) is a non-membrane bound structure composed of proteins and nucleic acids found within the nucleus. Ribosomal RNA (rRNA) is transcribed and assembled within the nucleolus.
Malfunction of nucleoli can be the cause for several human diseases. Three major components of the nucleolus are recognized: the fibrillar centers (FC), the dense fibrillar component (DFC) and granular components (GC). Another structure identified within many nucleoli, (particularly in plants) is a clear area in the center of the structure referred to as a nucleolar vacuole |
|
|
Describe the structure and function of the nuclear pore complex
|
Nuclear pores are large protein complexes that cross the nuclear envelope.
The proteins that make up the nuclear pore complex are known as nucleoporins. Nuclear pore complexes allow the transport of water-soluble molecules across the nuclear envelope. This transport includes RNA and ribosomes moving from nucleus to the cytoplasm and proteins (such as DNA polymerase and lamins), carbohydrates, signal molecules and lipids moving into the nucleus. It is notable that the nuclear pore complex (NPC) can actively conduct 1000 translocations per complex per second. Although smaller molecules simply diffuse through the pores, larger molecules may be recognized by specific signal sequences and then be diffused with the help of nucleoporins into or out of the nucleus. This is known as the RAN cycle. Each of the eight protein subunits surrounding the actual pore (the outer ring) projects a spoke-shaped protein into the pore channel. The center of the pore often appears to contain a plug-like structure. It is yet unknown whether this corresponds to an actual plug or is merely cargo caught in transit. |
|
|
Describe the organization of chromatin structure.
|
Chromatin is the combination of DNA, histone, and other proteins that make up chromosomes. It is found inside the nuclear envelope of eukaryotic cells. It is divided between heterochromatin (condensed) and euchromatin (extended) forms. The functions of chromatin are to package DNA into a smaller volume to fit in the cell, to strengthen the DNA to allow mitosis and meiosis, and to control gene expression and DNA replication. Changes in chromatin structure are affected by chemical modifications of histone proteins, such as methylation and acetylation, and by other DNA-binding proteins.
In simple terms, there are three levels of chromatin organization (Fig. 1): 1.DNA wraps around histone proteins forming nucleosomes - the "beads on a string" structure. 2.A 30 nm condensed chromatin fiber consisting of nucleosome arrays in their most compact form. 3.Higher-level DNA packaging into the metaphase chromosome. |
|
|
Predict cellular activity based on the chromatin structure
|
Condensed - Interphase
Extended - Immediately before M Phase Chromatin concentration, formation of kinetochores - Prophase Chromatids at center - Metaphase Sister Chromatids seperated - Anaphase Sister Chromatids at opposite poles - Telophase Two joined daughter cells - Cytokinesis |
|
|
List and describe the sequence of events occuring in interphase
|
S phase
– Replication of DNA – Replication of centrosome G2-to-M transition – two centrosomes separate – move to opposite poles – orientation determines the |
|
|
List and describe the sequence of events occurring in mitosis
|
M Phase
– Asexual Reproduction Production of 2 genetically identical daughter cells – Dynamic Sequence • Chromosomes • Cytoskeleton • Centrosomes – Cell Division • Nuclear division (karyokinesis karyokinesis) • Cytoplasmic division (cytokinesis cytokinesis) – 5 Stages • Prophase • Prometaphase • Metaphase • Anaphase • Telophase |
|
|
Describe the structure and function of the centromere
|
Centromere––Direct movement of chromosome ––Centric ––Persists throughout interphaseinterphase••
|
|
|
Describe the structure and function of the kinetochore
|
Kinetochore––Large protein complex (>80 proteins)––Forms near the centromere––Attach chromosome to mitotic spindle
|
|
|
Describe the structure and function of the centrosome
|
Schematic of typical animal cell, showing subcellular components. Organelles:
(1) Nucleolus (2) Nucleus (3) Ribosomes (little dots) (4) Vesicle (5) Rough endoplasmic reticulum (ER) (6) Golgi apparatus (7) Cytoskeleton (8) Smooth ER (9) Mitochondria (10) Vacuole (11) Cytoplasm (12) Lysosome (13) Centrioles within CentrosomeIn cell biology, the centrosome is an organelle that serves as the main microtubule organizing center (MTOC) of the animal cell as well as a regulator of cell-cycle progression. Centrosomes are composed of two orthogonally arranged centrioles surrounded by an amorphous mass of protein termed the pericentriolar material (PCM). The PCM contains proteins responsible for microtubule nucleation and anchoring including γ-tubulin, pericentrin and ninein. In general, each centriole of the centrosome is based on a nine triplet microtubule assembled in a cartwheel structure, and contains centrin, cenexin and tektin. |
|
|
Describe the structure and function of the mitotic spindle
|
Microtubules
–Overlap –Kinetochore –Astral Motor Proteins –Dynein –Kinesin |
|
|
Describe the role of cyclins and cyclin-dependent kinases in cell cycle regulation.
|

A family of proteins that control the progression of cells through the cell cycle by activating cyclin-dependent kinase (Cdk) enzymes by cyclically varying their concentration. Upon phosphorylation, a cyclin/CDK complex activates the CDK active site, forming maturation-promoting factor (MPF). MPFs activate other proteins through phosphorylation and these other proteins are responsible for specific events such as microtubule formation and chromatin remodeling.
|
|
|
List and describe the checkpoints of cell cycle regulation.
|
G1 Checkpoint (Restriction Point) – located at the end of the cell cycle’s G1 phase, just before S phase. Decides whether the cell should divide or enter G0.
G2 Checkpoint – located at the end of G2, triggering M Phase if the cell’s DNA is not damaged and the cell is ready for mitosis. Metaphase Checkpoint (Spindle Checkpoint) – occurs in metaphase when all chromosomes should have aligned at the mitotic plate, beginning Anaphase. |
|
|
Describe the role of the anaphase-promoting complex in mitosis and cell cycle regulation
|
Once the Metaphase Checkpoint is passed (chromosmes are at mitotic plate under bipolar tension) cyclin B is degraded. This ensures that it no longer inhibits the anaphase-promoting complex, which is now free to break down securin. This protein’s function is to inhibit separase, the protein responsible for the separation of the sister chromatids.
|
|
|
List and describe the sequence of events occuring in meiosis.
|
Meiosis I – separation of chromosomes from diploid to haploid.
Prophase I – The nucleoli and the nuclear envelope disappear. Exchange of DNA in homologous recombination, involving chromosomal crossover of chiasmata. Metaphase I – Homologous pairs move to metaphase plate as microtubules from both centrioles attach at the kinetochores. Anaphase I – Kinetochore microtubules shorten, separating the homologous chromosomes forming two haploid sets, each containing a pair of sister chromatids. Telophase I – Ends when the chromosomes arrive at the poles, uncoiling back into chromatin. Cytokinesis then occurs, creating two daughter cells which are attached. Interphase (Interkinesis) II Meiosis II – production of four haploid cells from the two produced in Meiosis I. Prophase II – The nucleoli and the nuclear envelope again disappear. Chromatids again shorten and thicken. Centrioles move to poles. Metaphase II – Chromosomes aligned on a new metaphase plate, perpendicular to the first. Anaphase II – Centromeres are cleaved, allowing microtubules to pull the sister chromatids apart and move them to opposite poles. Telophase II – uncoiling and lengthening of the chromosomes and disappearance of the spindle. Nuclear envelope reforms producing four daughter cells. |
|
|
List and describe the 5 stages of of Prophase I in meiosis.
|
Leptotene
– Individual chromosomes, consisting of sister chromatids, condense & connect to each other - Homologous chromosomes are each made of matching maternal and paternal components Zygotene - Pairing of homologous chromosomes (Synapsis) - Formation of a Synaptnemal Complex binding chromosomes together Pachytene - Completion of Synapsis and transposition of DNA strands between non-sister chromatids at chiasmata Diakinesis - Homologous chromosomes condense - Nucleolus disappears and nuclear envelope disintegrates Diplotene - Synaptonemal complex breaks down, homologous chromosomes separate - Chiasmata created during Pachytene may be noticeable |
|
|
List and describe the two events in meiosis that increase genetic diversity
|
Random alignment on the metaphase plate exposes different sides to centrioles
Crossing over of chromatids at chiasmata |
|
|
Compare and contrast necrosis and apoptosis
|
Necrosis - Pathological acute cell injury. Cell is unable to maintain homeostasis or maintain its plasma membrane. Cell contents are released causing surrounding tissue damage and inflammation.
Apoptosis - Physiological programmed cell death. Dell maintains its plasma membrane as the entire cell, including the nucleus, condense. Does not cause surrounding tissue damage or inflammation. |
|
|
Describe the intrinsic and extrinsic apoptosis pathways
|
Extrinsic
Ligand binds to death receptor, recruiting death domain adaptor proteins. Activation of initiator then effector caspases Apoptosis Intrinsic Death signal (e.g. Oxidative stress) releases cytochromec from the mitochondria. Formation of apoptosome and activation of caspase9 and effector caspases Apoptosis |
|
|
Define benign, melignant and metastasis
|
Benign - a condition that is harmless
Malignant - a severe and progressively worsening disease Metastasis - the spread of a disease from one organ or part to another non-adjacent organ or part. |
|
|
Describe the classification of cancer cells including carcinoma, sarcoma and leukemia
|
Carcinoma - an invasive malignant tumor consisting of transformed epithelial cells.
Sarcoma - a cancer that arises from transformed connective tissue cells. Leukemia - a type of cancer of the blood or bone marrow characterized by an abnormal increase of white blood cells. |
|
|
Define oncogene and tumor suppressor gene
|
Oncogene - a gene that has the potential to cause cancer.
Tumor Suppressor Gene - an anti-oncogene, a gene that protects a cell from one step on the path to cancer. |
|
|
Describe the correlation between cell cycle control and cancer
|
Cell cycle checkpoints are control mechanisms that ensure the fidelity of cell division in eukaryotic cells. Removal of these checkpoints constitutes cancer.
|
|
|
Describe the role of the retinoblastoma (Rb) and p53 tumor suppressor genes in the cell cycle and cancer
|
The retinoblastoma protein (pRB or Rb) is a tumor suppressor protein that regulates the cell cycle. It is also a recruiter of several chromatin remodelling enzymes such as methylases and acetylases. Should an oncogenic protein bind and inactivate pRb, this can lead to cancer.
p53 (also known as protein 53 or tumor protein 53), is a tumor suppressor protein that in humans is encoded by the TP53 gene. p53 is important in multicellular organisms, where it regulates the cell cycle and, thus, functions as a tumor suppressor that is involved in preventing cancer. |
|
|
Explain the role of telomerase in cell senescence and tumor growth
|
Telomerase is an enzyme that adds DNA sequence repeats ("TTAGGG" in all vertebrates) to the 3' end of DNA strands in the telomere regions. Telomerase is a reverse transcriptase that carries its own RNA molecule, which is used as a template when it elongates telomeres, which are shortened after each replication cycle.
|
|
|
Describe tumor growth and progression
|
The growth of neoplastic cells exceeds and is not coordinated with that of the normal tissues around it. The growth persists in the same excessive manner even after cessation of the stimuli. These cells are presumed to be clonal - that is, they are descended from a single progenitor cell. Sometimes, the neoplastic cells all carry the same genetic or epigenetic anomaly which becomes evidence for clonality.
|
|
|
Explain how abnormal cell division can result in clinical syndromes
|
A series of growth disorders can occur at the cellular level and these consequently underpin much of the subsequent course in cancer, in which a group of cells display uncontrolled growth and division beyond the normal limits, invasion (intrusion on and destruction of adjacent tissues), and metastasis (spread to other locations in the body via lymph or blood).
|
|
|
describe what is meant by karyotyping and cytogenetic studes and explain their clinical significance.
|
Karyotyping is the study of the number and appearance of chromosomes in a eukaryotic cell.
Cytogenetics is concerned with the study of the structure and function of chromosomes. Differentiation and enumeration of chromosomes relate aberrant chromosomes or chromosome number to congenital disorders such as Down’s syndrome. |
|
|
Define and compare/contrast histopathology versus cytopathology
|
Cytopathology - Cytopathology - a branch of pathology that studies and diagnoses diseases on the cellular level.
Histopathology - the microscopic examination of tissue in order to study the manifestations of disease. |
|
|
List the potential outcomes for a cell exposed to a change in its environment (e.g. successful cell response vs unsuccessful cell response)
|
I am not able to answer this question.
|
|
|
Name at least 3 basic types of pathological stimuli that may result in cell injury.
|
Infection
Toxin Trauma Thermal Injury Nutritional Immunilogical mechanisms |
|
|
Describe the main factors that determine a cell's fate when exposed to an injurious stimulus.
|
If the stimulus is persistent or of sufficient magnitude, then irreversible injury may develop.
The severity of the injury may also depend on the specific properties of the cell as in selective vulnerability. Cell death occurs when there is an irreversible loss of integrated cellular function. |
|
|
Define cellular degeneration and list 3 common structural changes
|
Cellular degeneration is a result of sub-lethal damage.
Changes include: mitochondrial swellings: at first, spaces of vacuoles develop within the mitochondrion, distorting the cristae; eventually the cristae will be destroyed, which will permanently damage the mitochondrioin swelling of ER and loss of ribosomes attached to RER cell appears to swell and beocome pale (cloudy swelling and hydropic degeneration) |
|
|
Describe the histological appearance of cloudy swelling and hydropic degeneration, and explain the cause of these changes
|
Microscopically, these reversible changes caused by swelling of the organelles is reflected in cellular swelling, paleness of cytoplasm and development of small intracellular vacuoles, giving rise to the widely used descriptive terms cloudy swelling and hydropic degeneration.
|
|
|
Describe the histological appearance of fatty degeneration. List the types of cells in which it occurs and explain the cause of this change.
|
the process describing the abnormal retention of lipids within a cell. It reflects an impairment of the normal processes of synthesis and elimination of triglyceride fat. Excess lipid accumulates in vesicles that displace the cytoplasm.
|
|
|
Explain why hepatocytes appear vacuolated in H&E stained sections from fatty degeneration of the liver
|
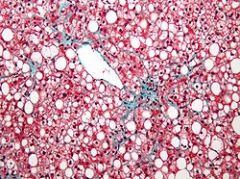
The liver is the primary organ of lipid metabolism it is most often associated with steatosis.
The clear vacuoles contained lipid in life; however, histological fixation caused it to be dissolved and hence only empty/clear spaces are seen. |
|
|
Name the mitochondrial enzyme which is released into cytoplasm in apoptosis from many different causes.
|
SMACs (second mitochondria-derived activator of caspases) are released into the cytosol following an increase in permeability. SMAC binds to inhibitor of apoptosis proteins (IAPs) and deactivates them, preventing the IAPs from arresting the apoptotic process and therefore allowing apoptosis to proceed.
|
|
|
Describe the enzyme sysgtem which is the final effector mechanism for apoptosis.
|
Several proteins are involved, but two main methods of regulation have been identified: targeting mitochondria functionality, or directly transducing the signal via adaptor proteins to the apoptotic mechanisms. Another extrinsic pathway for initiation identified in several toxin studies is an increase in calcium concentration within a cell caused by drug activity, which also can cause apoptosis via a calcium binding protease calpain.
|
|
|
List 5 common injurious stimuli whcih can trigger apoptosis
|
by ligation of cell surface receptors, by DNA damage as a cause of defects in DNA repair mechanisms, treatment with cytotoxic drugs or irradiation, by a lack of survival signals, contradictory cell cycle signalling or by developmental death signals.
|
|
|
Explain which structure/organelle in the cell is damaged leading to dysplasia and neoplasia
|
Rough Endoplasmic Reticulum. This is where proteins are made.
|
|
|
List examples of physiological processes involving apoptosis
|
Any loss of macro structures such as webbed fingers, tails, etc. Children lose something like 30 billion cells a day to apoptosis.
|
|
|
Describe the process of autodigestion or autolysis of the cell and the oganelle primarily responsible.
|
autolysis, more commonly known as self-digestion, refers to the destruction of a cell through the action of its own enzymes. It may also refer to the digestion of an enzyme by another molecule of the same enzyme.
Autolytic cell destruction is uncommon in living adult organisms and usually occurs in injured cells or dying tissue. Autolysis is initiated by the cells' lysosomes releasing the digestive enzymes they contain out into the cytoplasm. |
|
|
Define and compare/contrast pyknosis, karyorrhexis and karyolysis
|
Pyknosis (Karypyknosis) - irreversible condensation of chromatic in the nucleus prepatory to apoptosis or necrosis.
Karyorrhexis - Fragmentation of the nucleus before cell death Karyolysis - Complete dissolution of the chromatin matter of a dying cell due to the activity of DNase. Occurs mainly as a result of necrosis, while in apoptosis after karyorrhexis the nucleus usually dissolves into apoptotic bodies. |
|
|
Describe the structural changes that occur in cells after apoptosis is triggered
|
These changes include blebbing, loss of cell membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation.
|
|
|
Describe the appearance of apoptotic bodies
|
Small, sealed membrane vesicles which dye uniformly.
|

