![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
|
What is the general energy production and utilization Scheme? |
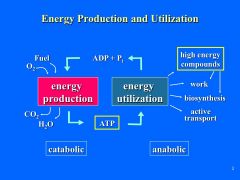
|
|
|
Where are reducing agents NADH + H^+ and FAD(2H) primarily used? |
Used primarily in energy production (e.g. production of ATP in mitochondria via electron Transfer coupled to ATP synthase |
|
|
Where are NADPH + H^+, UDP-Glucose and UDP Galactose primarily used? |
They are primarily used in biosynthetic pathways (e.g. Fatty Acid Synthesis) |
|
|
Name 4 high energy compounds that are rich in Phosphates |
1. Nucleoside Triphosphate (ATP) 2. Creatine Phosphate (Phosphocreatine) 3. 1,3-biophosphoglycerate 4. Phosphoenolpyruvate |
|
|
Name 2 High energy compounds that are rich in thioesters Name the two associated activated intermediates |
1. Acetyl-CoA 2. Succinyl-CoA Activated Intermediates 1. UDP-Glucose 2. UDP-Galactose |
|
|
1. If the Gibbs free energy of reactants is greater than the free energy of the products then what will occur? |
If the free energy of the “reactants” is greater than the free energyof the “products”, then the reaction will tend to proceed toward“products” and the free energy change will be negative. |
|
|
If the Gibbs free energy of the reactants is equal to the free energy of the products, then what will occur? |
If the free energy of the “reactants” is equal to the free energy of the“products”, then the reaction is at equilibrium and the free energy changeis 0. |
|
|
If the free energy of the reactants is less than the free energy of the products, then what will occur? |
If the free energy of the “reactants” is less than the free energy ofthe “products”, then the reaction will tend to proceed toward “reactants”,and the free energy change will be positive.
|
|
|
What is the difference between equilibrium and a steady state in regard to Gibbs free energy? |
A <----> B At equilibrium [B] is constant and the free energy change is 0. An example of this is bicarbonate in extracellular fluid. H2CO3 <-----> H^+ + HCO3^- (HCO3^- is essentially constant A---->B---->C In a steady state [A] and [C] are changing, but [B] is remaining relatively constant. Even though [B] is constant the reactions are not at equilibrium and the free energy change is not equal to zero. An example of this is CO2 (tissues) ---->CO2 (blood) -----> CO2 (lungs) [CO2 (blood) is constant. |
|
|
What is Standard Free Energy Based on? |
It is based on energy per mol under standard conditions |
|
|
If 1 mol reactant contains more energy than 1 mol of product, then the standard free energy change will be what? |
If 1 mol “reactant” contains more energy than 1 mol “product”, then thestandard free energy change is less than 0. Under “standard conditions”(1M “reactant”; 1M “product”) the reaction would proceed from left toright (toward equilibrium).
|
|
|
If 1 mol reactant contains less energy than 1 mol of product, then the standard free energy change will be what?
|
If 1 mol “reactant” contains less energy than 1 mol “product”, then thestandard free energy change is greater than 0. Under “standardconditions” (1M “reactant”; 1M “product”) the reaction would proceedfrom right to left (toward equilibrium).
|
|
|
If 1 mol reactant contains the same energy as 1 mol of product, then the standard free energy change will be what?
|
If 1 mol “reactant” contains the same amount of energy as 1 mol“product”, then the standard free energy change is 0. Under “standardconditions” (1M “reactant”; 1M “product”) the reaction would be atequilibrium.)
|
|
|
What is the reaction quotient (Q) at equilibrium? |
Qeq = [B]eq/[A]eq (Keq) |
|
|
If Q < Keq then what will the change in free energy be and how will the reaction proceed? |
If the reaction quotient (Q) is less than the equilibrium constant(Keq), then the Gibb’s free energy will be negative and the reactionwill tend to proceed from left to right.
|
|
|
If Q = Keq then what will the change in free energy be and how will the reaction proceed? |
If the reaction quotient (Q) is equal to the equilibrium constant(Keq), then the reaction is at equilibrium, the Gibb’s free energy will be zero, and the reaction will proceed in both directions at thesame rate.
|
|
|
If Q > Keq then what will the change in free energy be and how will the reaction proceed? |
If the reaction quotient (Q) is greater than the equilibrium constant(Keq), then the Gibb’s free energy will be positive and the reactionwill tend to proceed from right to left.
|
|
|
What is the reaction quotient (Q)? |
Q = [B]/[A] |
|
|
What is Keq? |
The "K" in Keq stands for "Constant". The "eq" means that the reaction is at equilibrium. Very roughly, Keq tells you the ratio of Products/Reactants for a given reaction at equilibrium. at a certain temperature.
|
|
|
What is the equation that relates change in Gibbs free energy to Q and Keq? |
Change in Gibbs Free Energy = (RTln) (Q/Keq)
|
|
|
What is the equation that relates standard free energy change to Q and Keq? |
Change in Standard Free Energy = -(RT)(ln)(Keq) |
|
|
What is the equation that relates change in Gibbs free energy to change in standard free energy and the reaction quotient? |
change in Gibbs Free Energy = Change in Standard Free energy + (RT)(ln)(Q) |
|
|
The change in Gibbs free energy for coupled reactions is the same as what? Provide an example. |
The change in Gibbs free energy for coupled reactions is the sum of the change in Gibbs free energy of the individual reactions. For example: glucose + ATP <----> Glucose-6-P + ADP (first step in glycolysis) 1. ATP + H2O <----> ADP + Pi = -7.3 kcal/mol 2. Glucose + Pi <------> Glucose-6-P + H2O = 3.3 kcal/mol 1+2 = Glucose + ATP <-----> Glucose-6-P + ADP = -4kcal/mol |
|
|
What is the equation that connects change in standard free energy to Redox potentials? |
Change in standard free energy = -nf(Change Eo) n=number of moles of electrons transferred per mole of reactants f = Faraday constant (23.06 kcal/Volts) Change Eo = Redox potential of the oxidation reduction reaction |
|
|
explain how to calculate the change in standard free energy that occurs when ethanol is oxidized to acetaldehyde |

|

