![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
Radial Distribution function?
|
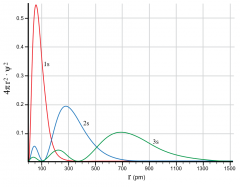
describes probabilyt of finding an electron at a given radius (in all directions) |
|
|
Slaters rule?
|
For electron in shell n-1, s = 0.85 For electron in shell <n-1, s = 1 Where s = shielding effect. S =∑s |
|
|
Effective nuclear charge?
|
where Z = nuclear charge S= shielding constant |
|
|
Trends in Effective nuclear charge?
|
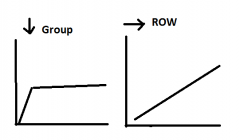
across row: Zeff increases - additional protons, poorly shielding additional electrons s = 0.35 down group, Zeff increases for first few elements then remains constant. |
|
|
Trends in orbital energies?
|
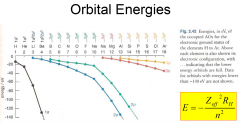
-Down group energies become less negative, n-increases, Zeff-same -across row enegies become more negative Zeff-increases, n-constant |
|
|
Atomic Radii trends?
|
across row radii decreases - Zeff increases, valence electrons more attracted to nucleus. |
|
|
Atomic radii exceptions?
|
Ga - 10 extra protons, 10 extra non-fully shielding electrons therefore Zeff increases therefore smaller radius |
|
|
Ionisation energy (definition)
|
The energy required to remove an electron from a gas-phase atom |
|
|
Ionisation energy trends?
|
across Row IE (generally) increases- Zeff increases, |
|
|
Ionisation energy exceptions
|
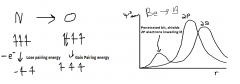
|
|
|
Electronegativity definition?
|
|
|
|
Electronegativity trends?
|
Increases across a period - Zeff increases, Decreases down a group, |
|
|
Metallic element characteristics?
|
Readily give up electrons (low ionisation energy) Metallic bonding Delocalised electrons Conduct heat and electricity |
|
|
Non-metallic elements characteristics?
|
High electron affinity Covalent bonding insulators Low melting and boiling points |
|
|
Metalloid elements characteristics?
|
intermediate electronegativity Characteristics of bot hmetals and non-metals |
|
|
ionisation (eq)
|
M(g) -> M(g)⁺ + e⁻ |
|
|
Standard reduction potential E₀ (eq)
|
∆G = -nFE₀ n = number of electrons F = faraday constant ∆G = gibbs free energy (wants to be negative for a reaction to go forward rather than back. therefore negative E₀ is a disfavoured process |
|
|
What sort of compounds do group 1 halides form
|
ionic compounds |
|
|
What causes ionic compounds to form? |
Large electronegativity differences between atoms |
|
|
trends in ∆H of formation for group 1 halides. |
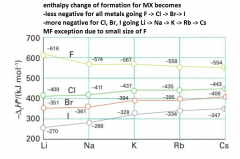
|
|
|
Born Haber Cycle
|
.eyeoieuoaiaoue |

