![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
How does the cytoplasm of a necrotic cell stain with H&E compared to a healthy one? Why?
|
Deep pink compared to healthy pale pink.
More avid binding of eosin to denatured cytoplasmic proteins |
|
|
What are the 3 cytoplasmic changes to note in a necrotic cell?
|
Eosinophilia
Vacuolization (enzymatic digestion of organelles) calcification |
|
|
Compare what you're looking for when you look at necrotic cells vs necrotic tissues.
|
Necrotic cells: cytoplasmic changes and nuclear changes
Necrotic tissues: looking for patterns in a mass of cells (coagulative, liquefactive, or caseous necrosis) |
|
|
What nuclear changes may you see in necrotic cells?
|
Pick one:
karyolysis pyknosis karyorrhexis Until 2 days after onset of necrotic cell death-- nucleus simply disappears |
|
|
What is karyolysis?
|
DNA-ases have degraded DNA. When stained with H&E, the nucleus is pale blue rather than a health dark blue.
|
|
|
What is pyknosis?
|
Nucleus shrinks and DNA condensates- H&E stains very dark blue (darker than healthy dark blue)
|
|
|
What is karyorrhexis?
|
nucleus undergoes framentation
|
|
|
How long before necrotic cell's nucleus disappears?
|
2 days
I bet L&O SVU labs know that |
|
|
You suspect tissue death from hypoxia/ischemia- what necrotic tissue pattern do you expect to see microscopically? Describe.
|
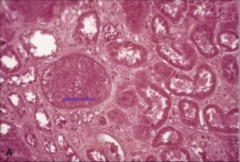
Coagulative necrosis
Outlines of every necrotic cell preserved and recognizable-- retained because denaturation of cellular proteins (ENZYMES) predominates. No autolysis. |
|
|
You suspect tissue death from a focal bacterial infection- what necrotic tissue pattern do you expect to see microscopically? Describe.
|
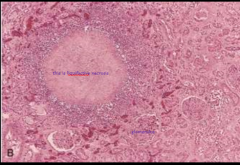
Liquefactive necrosis
Cells appear liquefied due to digestion and lysis by enzymes coming from either the cells' own lysosomes (autolysis) or from local WBC (activated leukocytes; heterolysis) |
|
|
You suspect a pt has TB- what necrotic tissue pattern do you expect to see microscopically on biopsy? Describe.
|
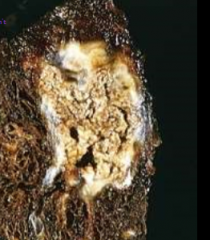
caseous necrosis
Tissue GROSSly appears "cheesy" (image is gross appearance, not microscopic) Microscopically: necrotic focus of fragmented cells and granular cellular debris surrounded by border of granulomatous inflammation (explained in different lecture) |
|
|
When they say "programmed cell death, type I" or "type II" what are those?
|
type I = apoptosis
type II = autophagy |
|
|
What's the general overview of the apoptotic process?
|
Initiation phase: intracellular "suicide program" gets activated (either intrinsically or extrinsically)
Execution phase: Activates intracellular enzymes -> degrade nucleus and cytoplasmic proteins |
|
|
In what kinds of situations would cells apoptose?
|

For physiologic reasons: during embryogenesis/development, replacing immature cells with /matoor/ cells. Hormonal dependent involution- menstruation.
For pathologic reasons: TNF -> in tumors. Viral diseases (hepatitis). Etc, etc, etc. |
|
|
What is the goal of the initiation phase?
|
Activate the "initiator caspase cascade"
|
|
|
Initiation phase: Intrinsic pathway
aka? What is the end game here to get that caspase cascade going? |
mitochondrial pathway
Make mitochondria spill cytochrome c to get apoptosomes formed which bind and activate initiator caspase 9 |
|
|
How is the intrinsic apoptotic pathway initiated?
|
Certain stressors (like radiation or deprivation of relevant hormone) activate intracytoplasmic sensor proteins-- a subfamily of Bcl-2 called "BH3-only" which includes Bim, Bid, and Bad
|
|
|
What role do Bim, Bid, Bad and related BH3-only proteins play?
|
These are sensor proteins. When their spidey-senses tingle, they activate Bax and Bak (pro-apoptotic proteins)
|
|
|
What do Bax and Bak do?
|
They polymerize into oligomers and insert themselves in the mitochondrial membrane making channels to release proteins (most notably cytochrome c) from the inner mitochondrial membrane.
They also inhibit anti-apoptotic proteins including Bcl-x and certain Bcl-2 proteins |
|
|
Bonus question: we learned about Bcl-2 before-- what was it?
|
An oncogene that gets upregulated in follicular lymphoma after it's put behind an immunoglobulin promoter in B lymphocytes. That's a slow growing cancer since the oncogene is for not dying instead of explosive growth.
|
|
|
What happens after cytochrome c gets released from mitochondria?
|
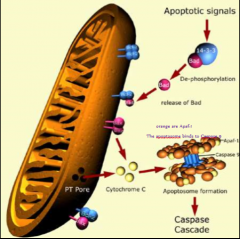
It binds to Apaf-1 (APoptosis-Activating Factor-1) which forms a hexamer called The Apoptosome which binds and activates caspase-9 which activates other caspase-9 molecules and then initiator caspase cascade is off and running.
|
|
|
What happens in the extrinsic apoptosis initiation pathway?
What's another name for this pathway? |
death receptor pathway- cell has these receptors for apoptotic signal delivery. Examples: TNFR1 and Fas.
When ligands bind these it activates caspases 8 and 10 -> trigger others and initiator caspase cascade takes off. |
|
|
Ok. So you've activated the initiator caspase cascade. Now what?
|
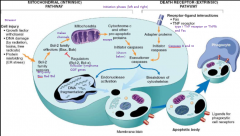
The cascade activates The Executioner caspases like caspase 3 and 6 -> degradation of DNA, nuclear proteins; cytoskeletal decoupling makes for blebbing -> death
|
|
|
What happens to apoptotic cells' remains?
|
Get phagocytosed by macrophages or other phagocytic cells
|
|
|
What type of inflammation do the apoptotic cells elicit?
|
Neither type- they don't elicit inflammatory response because of the rapidity of phagocytosis
|
|
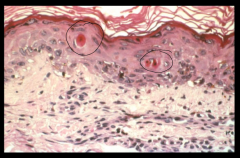
this is not necrosis- how do I know that? i.e. what are the morphological characteristics of apoptosis? 5
|
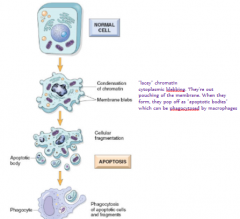
involves single cells or a small group of cells
minimal membrane damage nuclear chromatin condenses pronounced blebbing (membrane-bound apoptotic bodies) no inflammatory response |
|
|
When do you go the programmed cell death type II route?
|
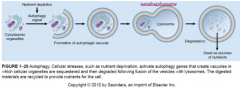
autophagy is for cell that are nutritionally deprived-- an autophagic vacuole with organelles inside fuses with a lysosome => autophagolysosome which digests the materials and releases them back into cytoplasm to provide nutrients in desperate situations
does that make this like ethical cannibalism? autophagy also has role in defense of viral/bacterial infections |
|
|
If the autophagy pathway gets upregulated too much it's bad. What types of diseases is it seen in? Name 2 specifically.
|
It destroys too many organelles -> cell death
Degenerative diseases of nerve and muscle Alzheimer's disease ALS (amyotrophic lateral sclerosis) |
|
|
What genes regulate autophagy?
|
Atg genes
|
|
|
What do they mean when they say it's unclear whether autophagy is the direct cause of cell death?
|
Is that the real problem - that just that pathway is going crazy in and of itself- as opposed to another stressor driving the death as well as the autophagy
|
|
|
Show time.
What are the 5 major ways necrosis and apoptosis differ? |

|

