![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
causes of hypoxia
|
1) high altitude
2) poor ventilation 3) CO poisoning 4) impaired mitochondrial function 5) severe anemia 6) local restriction of blood flow |
|
|
what is the effect of hypoxia?
|
decrease of ATP -> anaerobic glycolysis -> lactic acid -> \/ pH -> Na/K pump malfunction
|
|
|
What three indications are there that ischemic/hypoxic injury is irreversible?
|
1) profound membrane damage
2) permanent loss of ability to generate ATP 3) cytoskeletal abnormalities 4) glycine is lost -> membrane injury all lead to cell death - necrosis |
|
|
reperfusion injury
|
after irreversible injury, ROS are produced to remove damaged cells and surrounding healthy cells can be damaged.
|
|
|
what is the final and subsequently lethal effect of ischemic/hypoxic events that will ultimately cause cell death?
|
profound membrane damage
|
|
|
threshold dose
|
lowest dose at which a response occurs
|
|
|
subthreshold dose
|
no measurable response, "safe dose"
|
|
|
ceiling effect
|
plateau is reached at higher doses
|
|
|
xenobiotic
|
chemical which is found in an organism but which is not normally produced or expected to be present in it. most xenobiotics are lipophilic and are metabolized to hydrophilic substances.
|
|
|
phase I reactions
|
in liver, polar group is added to xenobiotic to create a more soluble metabolite. if not hydrophilic enough, then goes to phase II reactions. CYP P450 are heavily used here.
|
|
|
phase II reactions
|
metabolites conjugated with endogenous hydrophilic substances to increase hydrophilicity for clearance.
|
|
|
what type of tissue do xenobiotics love to sit in?
|
fatty tissue. they are naturally lipophillic, which is why the liver must go through phase I and phase II processes to increase hydrophilicity to allow elimination.
|
|
|
biotransformation
|
the overall term to describe the phase I and phase II conversion of lipophilic to hydrophilic compounds.
|
|
|
what is the significance of CYP1A1 gene?
|
people express this gene has an increased risk of lung cancer.
|
|
|
What activities work synergistically with smoking to compromise health?
|
1) asbestos worker
2) chronic drinker |
|
|
What percent of lung cancers are due to smoking?
|
90% !
|
|
|
Is increased risk of cancer isolated to lungs for smokers?
|
No! larynx, oral, esophageal, pancreas, bladder
|
|
|
what is the legal limit for DUI blood levels?
|
< 80 mg/dL
|
|
|
how is ethanol metabolized?
|
1) alcohol dehydrogenase in gastric mucosa & liver
2) CYP2E1 in liver and catalase in liver assists 3) acetaldehyde (toxic) is converted to acetic acid (water soluble) by aldehyde dehydrogenase |
|
|
what does alcohol do to the CNS?
|
acute CNS depressant
|
|
|
wernicke encephalopathy
|
thiamine (vit B1) deficiency - ataxia, conitive impairment, ophthalmoplegia, nystagmus
commonly grouped with korsakoff as "Wernicke-Korsakoff syndrome" |
|
|
korsakoff psychosis
|
thiamine (vit B1) deficiency - severe memory loss, confabulation, hallucination
commonly grouped with wernicke as "Wernicke-Korsakoff syndrome" |
|
|
effects of alcohol on liver
|
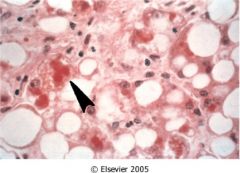
fatty liver (triglycerides in hepatocytes), acute hepatitis, cirrhosis
|
|
|
how does ethanol hurt the liver?
|
ethanol is directly toxic to hepatocytes caused by glutathione depletion, hepatocyte necrosis and fibrosis occurs around central veins, and cirrhosis
|
|
|
What is cirrhosis?
|
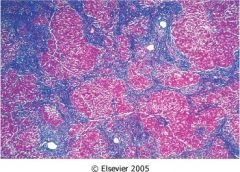
irreversible formation of micronodules of regenerating hepatocytes surrounded by collagen bands. this leads to portal hypertension, varices, GI bleeding
|
|
|
what are the cardiovascular effects of alcohol?
|
directly toxic to cardiac cells, dilated cardiomyopathy, hypertension caused by increased catecholamines
|
|
|
what are the cardiovascular benefits of alcohol?
|
moderate use (1-2 drinks/day) increases HDL, decreases platelet aggregation
|
|
|
fetal alcohol syndrome
|
microcephaly, dysmorphic facies, organ malformation, growth retardation, mental retardation
most common form of preventable mental retardation |
|
|
common cancers associated with alcohol use
|
oral cavity, pharynx, esophagus, liver
|
|
|
methanol metabolism
|
metabolized by alcohol dehydrogenase to formaldehyde and formic acid.
|
|
|
methanol poisoning clinical characteristics
|
metabolic acidosis, dizziness, vomiting, blindness, respiratory depression
|
|
|
methanol poisoning tx
|
ethanol - competitive inhibitor
|
|
|
ethylene glycol metabolism
|
component of antifreeze, metabolized by alcohol dehydrogenase to aldehydes, glycolate, oxalate, lactate
|
|
|
ethylene glycol poisoning clinical characteristics
|
metabolic acidosis, mental status, nausea, vomiting, acute renal failure due to oxalate crystal obstruction of renal tubules
|
|
|
ethylene glycol poisoning tx
|
ethanol - competitive inhibitor
|
|

|

|

