![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
199 Cards in this Set
- Front
- Back
|
Define rate of reaction and give units
|
The change in concentration of a reactant or product per unit time
Units: mol dm^-3 s^-1 |
|
|
How can a reaction take place?
|
Collisions between particles must have more energy than activation energy
|
|
|
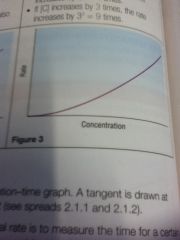
How to measure reaction rate
|
Draw a tangent on the concentration time graph and measure the gradient (which gives the rate)
|
|
|
Define initial rate of reaction
|
The change in concentration of a reactant, or product, per unit time at the start of the reaction when t=0
|
|
|
How to measure reaction rates
|
Acids or bases: measure pH changes by pH meter or titration
Production of gases: the change in volume or pressure or loss of mass of reactants Visual changes: formation of precipitate or colour changes |
|
|
Formula for gradient
|
Change in y / change in x
|
|
|
Define order
|
The power to which the concentration of a reactant is raised in the rate equation
|
|
|
Define rate constant, k
|
The constant that links the rate of reaction with the concentrations of the reactants raised to the powers of their orders in the rate equation
|
|
|
Define half life
|
The time taken for the concentration of the reactant to reduce by half
|
|
|
Half life and orders
|
Zero order: half life decreases with time
First order: half life is constant Second order: half life increases with time |
|

|
Zero order
|
|
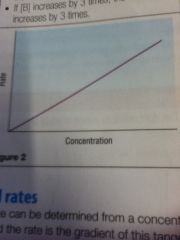
|
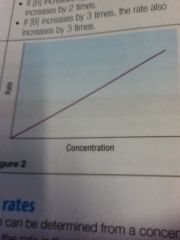
First order
|
|
|
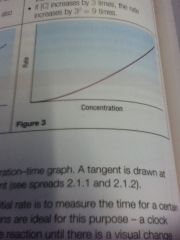
Second order
|
|
|
Rate concentration graphs allow you to determine
|
Orders with respect to reactants
|
|
|
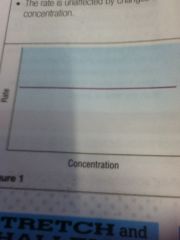
Zero order: rate is unaffected by changes in concentration
|
|
|
First order: rate increases by same amount as concentration
|
|
|
Rate increases by order squared
|
|
|
Measuring initial rate of reaction
|
Measuring the time for a certain amount if product to be formed: a clock reaction.
Keep one reactant constant and change the other one - then repeat the other way round Initial rate is proportional to 1/t |
|
|
The rate constant, k
|
Fast reaction has a large k
Slow reaction has a small k |
|
|
Effect of temperature on rate constant
|
Rate constant increases with increasing temperature, usually exponentially
|
|
|
The rate determining step definition
|
The slowest step in the reaction mechanism of a multi step reaction
|
|
|
Rate equations and rate determining step
|
Reactants in the rate equation MUST be in the rate determining step
Then balance by producing two equations, usually with an intermediate (formed in one step of the multi step reaction but used up in subsequent steps) |
|
|
Define dynamic equilibrium
|
When the rate of the forward reaction is the same as the rate of reverse reaction.
The concentrations of reactants and products remain the same |
|
|
The equilibrium constant, Kc and units
|
Products over reactants
Mol dm^-3 |
|
|
The equilibrium constant, Kc and units
|
Products over reactants
Mol dm^-3 |
|
|
Homogenous equilibrium
|
Equilibrium in which all the species making up the reactants and products are in the same physical state
|
|
|
The equilibrium constant, Kc and units
|
Products over reactants
Mol dm^-3 |
|
|
Homogenous equilibrium
|
Equilibrium in which all the species making up the reactants and products are in the same physical state
|
|
|
Heterogenous equilibrium
|
Equilibrium in which the species making up the reactants and products are in different physical states
|
|
|
The equilibrium constant, Kc and units
|
Products over reactants
Mol dm^-3 |
|
|
Homogenous equilibrium
|
Equilibrium in which all the species making up the reactants and products are in the same physical state
|
|
|
Heterogenous equilibrium
|
Equilibrium in which the species making up the reactants and products are in different physical states
|
|
|
Problems involving Kc
|
Write out Kc equation
Draw a table with initial, final and equilibrium moles Work out final moles of one substance (initial-equilibrium) Use reaction ratio to work out other final amounts, then subtract from initial to get equilibrium moles. Divide by volume to get concentration |
|
|
When Kc equals 1
|
Position of equilibrium is halfway between reactants and products
|
|
|
When Kc equals 1
|
Position of equilibrium is halfway between reactants and products
|
|
|
When Kc greater than 1
|
Reaction is product favoured
|
|
|
When Kc equals 1
|
Position of equilibrium is halfway between reactants and products
|
|
|
When Kc greater than 1
|
Reaction is product favoured
|
|
|
Kc less than one
|
Reaction is reactant favoured
|
|
|
When temperature increases in an exothermic reaction, Kc
|
Increases as the position of equilibrium moves to the right to increases products so reactant yield decreases
|
|
|
When temperature increases in an exothermic reaction, Kc
|
Increases as the position of equilibrium moves to the right to increases products so reactant yield decreases
|
|
|
An increase in temperature in an endothermic reaction means Kc will
|
Decrease as position of equilibrium moves to the left as yield of products decrease and reactants increase.
|
|
|
When temperature increases in an exothermic reaction, Kc
|
Increases as the position of equilibrium moves to the right to increases products so reactant yield decreases
|
|
|
An increase in temperature in an endothermic reaction means Kc will
|
Decrease as position of equilibrium moves to the left as yield of products decrease and reactants increase.
|
|
|
How is Kc affected by changes in pressure and concentration?
|
It's not! :)
|
|
|
When temperature increases in an exothermic reaction, Kc
|
Increases as the position of equilibrium moves to the right to increases products so reactant yield decreases
|
|
|
An increase in temperature in an endothermic reaction means Kc will
|
Decrease as position of equilibrium moves to the left as yield of products decrease and reactants increase.
|
|
|
How is Kc affected by changes in pressure and concentration?
|
It's not! :)
|
|
|
Changes in concentration
|
If the concentration of a reactant or product is altered, then system is no longer in equilibrium. So equilibrium must shift to restore Kc value.
By increasing unchanged concentration and decreasing changed concentration. |
|
|
When temperature increases in an exothermic reaction, Kc
|
Increases as the position of equilibrium moves to the right to increases products so reactant yield decreases
|
|
|
An increase in temperature in an endothermic reaction means Kc will
|
Decrease as position of equilibrium moves to the left as yield of products decrease and reactants increase.
|
|
|
How is Kc affected by changes in pressure and concentration?
|
It's not! :)
|
|
|
Changes in concentration
|
If the concentration of a reactant or product is altered, then system is no longer in equilibrium. So equilibrium must shift to restore Kc value.
By increasing unchanged concentration and decreasing changed concentration. |
|
|
If pressure doubled
|
The concentration of both reactants and products will also double. So it will decrease product concentration and increase reactant concentration to restore Kc
|
|
|
When temperature increases in an exothermic reaction, Kc
|
Increases as the position of equilibrium moves to the right to increases products so reactant yield decreases
|
|
|
An increase in temperature in an endothermic reaction means Kc will
|
Decrease as position of equilibrium moves to the left as yield of products decrease and reactants increase.
|
|
|
How is Kc affected by changes in pressure and concentration?
|
It's not! :)
|
|
|
Changes in concentration
|
If the concentration of a reactant or product is altered, then system is no longer in equilibrium. So equilibrium must shift to restore Kc value.
By increasing unchanged concentration and decreasing changed concentration. |
|
|
If pressure doubled
|
The concentration of both reactants and products will also double. So it will decrease product concentration and increase reactant concentration to restore Kc
|
|
|
A Brønsted - Lowry acid
|
A proton donor
|
|
|
Brønsted Lowry base
|
A proton acceptor
|
|
|
Brønsted Lowry base
|
A proton acceptor
|
|
|
Dibasic acid definition, example and equation
|
Each molecule can release two protons
E.g sulphuric acid H2SO4 ---> H+ + H2SO4- H2SO4- <---> h+ + HSO4- |
|
|
Reactions with carbonates
Equation of HCl and CaCO3 |
Forming a salt, carbon dioxide and water
2H+ + CaCO3 ---> Ca2+ + CO2 + H2O |
|
|
Reactions with carbonates
Equation of HCl and CaCO3 |
Forming a salt, carbon dioxide and water
2H+ + CaCO3 ---> Ca2+ + CO2 + H2O |
|
|
Reactions with bases
Equation of HNO3 and MgO |
Forms a salt and water
2H+ + MgO ---> Mg2+ + H2O |
|
|
Reactions with carbonates
Equation of HCl and CaCO3 |
Forming a salt, carbon dioxide and water
2H+ + CaCO3 ---> Ca2+ + CO2 + H2O |
|
|
Reactions with bases
Equation of HNO3 and MgO |
Forms a salt and water
2H+ + MgO ---> Mg2+ + H2O |
|
|
Reactions of acids and metals
|
Forms a salt and hydrogen
2H+ + Mg --> Mg2+ + H2 |
|
|
Reactions with carbonates
Equation of HCl and CaCO3 |
Forming a salt, carbon dioxide and water
2H+ + CaCO3 ---> Ca2+ + CO2 + H2O |
|
|
Reactions with bases
Equation of HNO3 and MgO |
Forms a salt and water
2H+ + MgO ---> Mg2+ + H2O |
|
|
Reactions of acids and metals
|
Forms a salt and hydrogen
2H+ + Mg --> Mg2+ + H2 |
|
|
Release of a proton from an acid
|
Usually when the acid is dissolved in water
E.g HCl + H2O ---> H3O+ + Cl- |
|
|
Reactions with carbonates
Equation of HCl and CaCO3 |
Forming a salt, carbon dioxide and water
2H+ + CaCO3 ---> Ca2+ + CO2 + H2O |
|
|
Reactions with bases
Equation of HNO3 and MgO |
Forms a salt and water
2H+ + MgO ---> Mg2+ + H2O |
|
|
Reactions of acids and metals
|
Forms a salt and hydrogen
2H+ + Mg --> Mg2+ + H2 |
|
|
Release of a proton from an acid
|
Usually when the acid is dissolved in water
E.g HCl + H2O ---> H3O+ + Cl- |
|
|
Acid base pairs
|
A pair of two species that transform into each other by gain or loss of a proton
The acid always has one more H+ |
|
|
Reactions with carbonates
Equation of HCl and CaCO3 |
Forming a salt, carbon dioxide and water
2H+ + CaCO3 ---> Ca2+ + CO2 + H2O |
|
|
Reactions with bases
Equation of HNO3 and MgO |
Forms a salt and water
2H+ + MgO ---> Mg2+ + H2O |
|
|
Reactions of acids and metals
|
Forms a salt and hydrogen
2H+ + Mg --> Mg2+ + H2 |
|
|
Release of a proton from an acid
|
Usually when the acid is dissolved in water
E.g HCl + H2O ---> H3O+ + Cl- |
|
|
Acid base pairs
|
A pair of two species that transform into each other by gain or loss of a proton
The acid always has one more H+ |
|
|
pH and H+
|
pH = -log H+
H+. = 10-pH |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Calculating pH for strong bases
|
Need to use Kw to find H+ which is Kw/OH-
Then work out pH |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Calculating pH for strong bases
|
Need to use Kw to find H+ which is Kw/OH-
Then work out pH |
|
|
A buffer solution definition
|
A mixture that minimises pH changes on addition of small amounts of acid or base
|
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Calculating pH for strong bases
|
Need to use Kw to find H+ which is Kw/OH-
Then work out pH |
|
|
A buffer solution definition
|
A mixture that minimises pH changes on addition of small amounts of acid or base
|
|
|
What is a buffer solution made up of?
|
A weak acid and its conjugate base
Either a weak acid and the salt of the weak acid such as methanoic acid and sodium methanoate |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Calculating pH for strong bases
|
Need to use Kw to find H+ which is Kw/OH-
Then work out pH |
|
|
A buffer solution definition
|
A mixture that minimises pH changes on addition of small amounts of acid or base
|
|
|
What is a buffer solution made up of?
|
A weak acid and its conjugate base
Either a weak acid and the salt of the weak acid such as methanoic acid and sodium methanoate |
|
|
The dissociations in the buffer
|

The weak acid partially dissociates
The salt dissociates completely forming the conjugate base and salt ion |
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Calculating pH for strong bases
|
Need to use Kw to find H+ which is Kw/OH-
Then work out pH |
|
|
A buffer solution definition
|
A mixture that minimises pH changes on addition of small amounts of acid or base
|
|
|
What is a buffer solution made up of?
|
A weak acid and its conjugate base
Either a weak acid and the salt of the weak acid such as methanoic acid and sodium methanoate |
|
|
The dissociations in the buffer
|

The weak acid partially dissociates
The salt dissociates completely forming the conjugate base and salt ion |
|
|
Equilibrium in a buffer solution
|
Contains a high concentration of undissociated weak acid and conjugate base. The high concentration of conjugate base pushes equilibrium to the LEFT so concentration of H+ is very small
|
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
On addition of acid
|
Concentration of H+ is increased and the A- reacts with H+. Equilibrium position moves to the LEFT to removed added H+
|
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Calculating pH for strong bases
|
Need to use Kw to find H+ which is Kw/OH-
Then work out pH |
|
|
A buffer solution definition
|
A mixture that minimises pH changes on addition of small amounts of acid or base
|
|
|
The dissociations in the buffer
|

The weak acid partially dissociates
The salt dissociates completely forming the conjugate base and salt ion |
|
|
The dissociations in the buffer
|
The weak acid partially dissociates
The salt dissociates completely forming the conjugate base and salt ion |
|
|
Equilibrium in a buffer solution
|
Contains a high concentration of undissociated weak acid and conjugate base. The high concentration of conjugate base pushes equilibrium to the LEFT so concentration of H+ is very small
|
|
|
Strong and weak acids
|
The strength of an acid is measured by extent of dissociation.
Strong acid has complete dissociation Weak acid has partial dissociation |
|
|
On addition of acid
|
Concentration of H+ is increased and the A- reacts with H+. Equilibrium position moves to the LEFT to removed added H+
|
|
|
On addition of added alkali
|
The small concentration of OH- ions react with H+ to form H2O. The HA dissociates, shifting equilibrium to the RIGHT to restore most of H+ ions that have reacted
|
|
|
Ka and units
|
H+ and A- / HA
Units are mol dm-3 A large Ka means a stronger acid |
|
|
Pka and Ka
|
A low value if Ka matches a high pKa
A high Ka means a low pKa The smaller the pKa, the stronger the acid |
|
|
The ionic product of water, Kw
|
At 25 degrees, it's equal to (H+) (OH-)
It controls the balance between H+ and OH- in all aqueous solutions |
|
|
The strength of a base and alkali definition
|
The measure of its dissociation to generate OH-
An alkali is a base that dissolves in water |
|
|
Calculating pH for strong bases
|
Need to use Kw to find H+ which is Kw/OH-
Then work out pH |
|
|
The dissociations in the buffer
|

The weak acid partially dissociates
The salt dissociates completely forming the conjugate base and salt ion |
|
|
What is a buffer solution made up of?
|
A weak acid and its conjugate base
Either a weak acid and the salt of the weak acid such as methanoic acid and sodium methanoate |
|
|
The dissociations in the buffer
|
The weak acid partially dissociates
The salt dissociates completely forming the conjugate base and salt ion |
|
|
Equilibrium in a buffer solution
|
Contains a high concentration of undissociated weak acid and conjugate base. The high concentration of conjugate base pushes equilibrium to the LEFT so concentration of H+ is very small
|
|
|
Calculations involving buffer solutions
|
(H+) = Ka * (HA)/(A-)
|
|
|
Calculations involving buffer solutions
|
(H+) = Ka * (HA)/(A-)
|
|
|
Carbonic acid - hydrogen carbonate ion buffer system
|
Controls blood pH so it's between 7.35 and 7.45
H2CO3 is the weak acid and HCO3- is weak base H2CO3 <----> H+ + HCO3- An increase in H+: removed by HCO3-, equilibrium shifts to LEFT An increase in OH-: removed by H2CO3, H+ reacts with OH- to form H2O and equilibrium huffs to RIGHT to restore H+ reacted. |
|
|
Calculations involving buffer solutions
|
(H+) = Ka * (HA)/(A-)
|
|
|
Carbonic acid - hydrogen carbonate ion buffer system
|
Controls blood pH so it's between 7.35 and 7.45
H2CO3 is the weak acid and HCO3- is weak base H2CO3 <----> H+ + HCO3- An increase in H+: removed by HCO3-, equilibrium shifts to LEFT An increase in OH-: removed by H2CO3, H+ reacts with OH- to form H2O and equilibrium huffs to RIGHT to restore H+ reacted. |
|
|
Define equivalence point
|
The point in a titration at which the volume of one solution has reacted exactly with the volume of the second solution
The VERTICAL SECTION if a titration curve |
|
|
Calculations involving buffer solutions
|
(H+) = Ka * (HA)/(A-)
|
|
|
Carbonic acid - hydrogen carbonate ion buffer system
|
Controls blood pH so it's between 7.35 and 7.45
H2CO3 is the weak acid and HCO3- is weak base H2CO3 <----> H+ + HCO3- An increase in H+: removed by HCO3-, equilibrium shifts to LEFT An increase in OH-: removed by H2CO3, H+ reacts with OH- to form H2O and equilibrium huffs to RIGHT to restore H+ reacted. |
|
|
Define equivalence point
|
The point in a titration at which the volume of one solution has reacted exactly with the volume of the second solution
The VERTICAL SECTION if a titration curve |
|
|
Choosing the indicator
|
Chosen so the pH value of the end point is as close as possible to the pH value of the titration's equivalence point
|
|
|
Calculations involving buffer solutions
|
(H+) = Ka * (HA)/(A-)
|
|
|
Carbonic acid - hydrogen carbonate ion buffer system
|
Controls blood pH so it's between 7.35 and 7.45
H2CO3 is the weak acid and HCO3- is weak base H2CO3 <----> H+ + HCO3- An increase in H+: removed by HCO3-, equilibrium shifts to LEFT An increase in OH-: removed by H2CO3, H+ reacts with OH- to form H2O and equilibrium huffs to RIGHT to restore H+ reacted. |
|
|
Define equivalence point
|
The point in a titration at which the volume of one solution has reacted exactly with the volume of the second solution
The VERTICAL SECTION if a titration curve |
|
|
Choosing the indicator
|
Chosen so the pH value of the end point is as close as possible to the pH value of the titration's equivalence point
|
|
|
Strong acid strong base curve
|
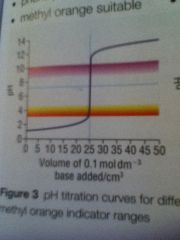
|
|
|
Calculations involving buffer solutions
|
(H+) = Ka * (HA)/(A-)
|
|
|
Carbonic acid - hydrogen carbonate ion buffer system
|
Controls blood pH so it's between 7.35 and 7.45
H2CO3 is the weak acid and HCO3- is weak base H2CO3 <----> H+ + HCO3- An increase in H+: removed by HCO3-, equilibrium shifts to LEFT An increase in OH-: removed by H2CO3, H+ reacts with OH- to form H2O and equilibrium shifts to RIGHT to restore H+ reacted. |
|
|
Define equivalence point
|
The point in a titration at which the volume of one solution has reacted exactly with the volume of the second solution
The VERTICAL SECTION of titration curve |
|
|
Choosing the indicator
|
Chosen so the pH value of the end point is as close as possible to the pH value of the titration's equivalence point
|
|
|
Strong acid strong base curve
|
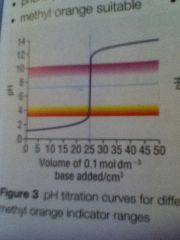
|
|
|
Weak acid - strong base curve
|
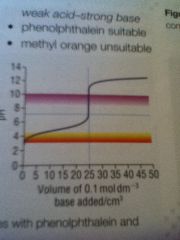
|
|
|
Calculations involving buffer solutions
|
(H+) = Ka * (HA)/(A-)
|
|
|
Carbonic acid - hydrogen carbonate ion buffer system
|
Controls blood pH so it's between 7.35 and 7.45
H2CO3 is the weak acid and HCO3- is weak base H2CO3 <----> H+ + HCO3- An increase in H+: removed by HCO3-, equilibrium shifts to LEFT An increase in OH-: removed by H2CO3, H+ reacts with OH- to form H2O and equilibrium huffs to RIGHT to restore H+ reacted. |
|
|
Define equivalence point
|
The point in a titration at which the volume of one solution has reacted exactly with the volume of the second solution
The VERTICAL SECTION if a titration curve |
|
|
Choosing the indicator
|
Chosen so the pH value of the end point is as close as possible to the pH value of the titration's equivalence point
|
|
|
Strong acid strong base curve
|
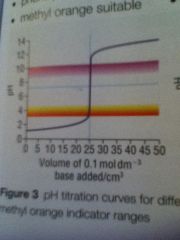
|
|
|
Strong acid weak base curve
|
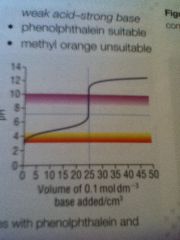
|
|
|
Weak acid strong base
|
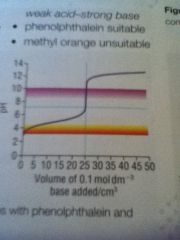
|
|
|
Weak act weak base curve
|
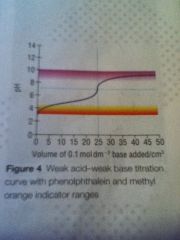
|
|
|
Weak act weak base curve
|
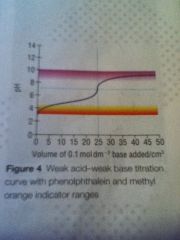
|
|
|
Define standard enthalpy of neutralisation
|
The enthalpy change that accompanies the neutralisation of an aqueous acid by an a aqueous base to form one mile of water, under standard conditions
|
|
|
Weak act weak base curve
|
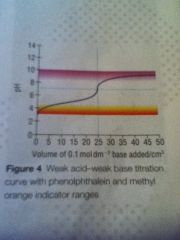
|
|
|
Define standard enthalpy of neutralisation
|
The enthalpy change that accompanies the neutralisation of an aqueous acid by an a aqueous base to form one mole of water, under standard conditions
|
|
|
Calculating the enthalpy change
|
Work out energy change, Q= m*c*temperature change
Work out amount in mol reacted Then divide Q by moles |

